X-ray Photoelectron Spectroscopy (XPS)
Home » Our Techniques » Spectroscopy » XPS-ESCA
X-Ray Photoelectron Spectroscopy (XPS) is also known as Electron Spectroscopy for Chemical Analysis (ESCA). X-Ray Photoelectron Spectroscopy is used to determine quantitative atomic composition and chemistry. It is a surface analysis technique with a sampling volume that extends from the surface to a depth of approximately 50-100 Å. XPS Spectroscopy can also be used for sputter depth profiling. This is useful to characterize thin films by quantifying matrix-level elements as a function of depth.
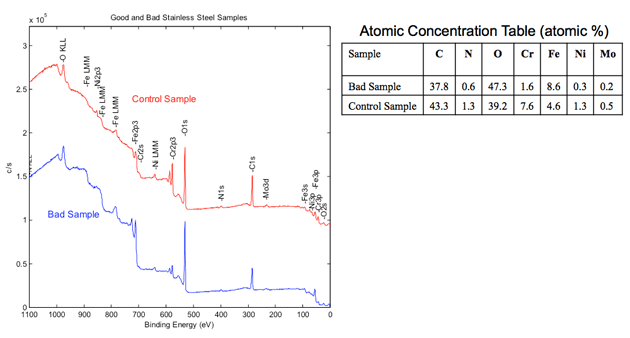
XPS Spectroscopy is an elemental analysis technique. This is unique in also providing chemical state information for the detected elements. A good use is distinguishing between sulfate and sulfide forms of sulfur. The process works by irradiating a sample with monochromatic X-rays. This results in the emission of photoelectrons whose energies are characteristic of the elements within the sampling volume.
X-rays penetrate a sample several microns deep and force electrons from the sample. Therefore, only electrons from the top 100 angstroms have enough energy to reach the XPS detector.
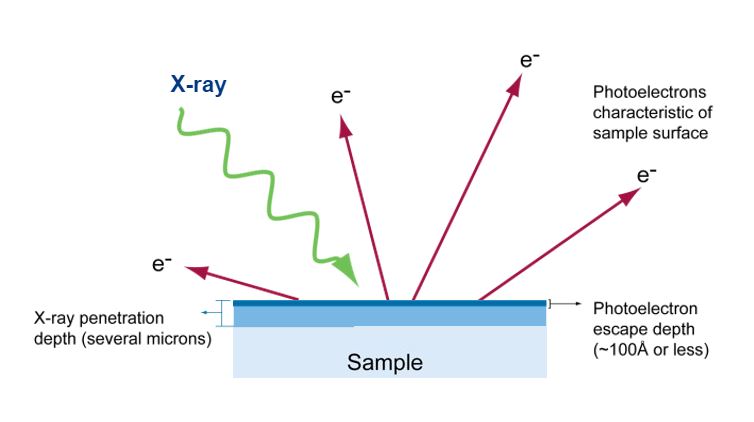
XPS measures the kinetic energy of the photoelectrons emitted from a sample exposed to monochromatic X-rays. The energies of the emitted electrons are characteristic of the elements present and also the chemical bonding or oxidation state of the elements. The number of Atoms is related to the quantity of emitted electrons. The penetration depth of X-rays can be as deep as several microns, but the photoelectrons created by direct ionization of the impacting X-rays escape only from the top few nm of the surface without losing energy.
Each orbital level of each element has a specific energy level and hence a specific binding energy required to remove the electron from the atom. The energy of the X-rays (hν), the kinetic energy of the emitted electrons (KE), and the binding energy (BE) of the electrons are related as follows:
BE = hν-KE-φ
φ is the work function of the spectrometer
Elemental analysis becomes possible by measuring the kinetic energy of photoelectrons. In addition, since the binding energy of elements varies slightly depending on their bonding state (and their chemical environment), information such as whether an element exists as a compound (and what kind) can be obtained from the amount of energy shift (chemical shift) at the photoelectron peak position.
Firstly, XPS Spectroscopy usually starts with a survey that looks at the full energy range scan at highest sensitivity. Therefore, we can identify and quantifies elements at surface. Secondly, we typically use high resolution XPS analysis to determine the bonding state where we use narrow scans at higher energy resolution. This can determine the chemical bonding state from peak position and peak shape. Finally, to determine a thin film composition, depth profile analysis is useful, as it looks at atomic composition.
EAG uses X-ray Photoelectron Spectroscopy in a variety of applications to help customers across a range of industries. For instance, with R&D, process development/improvement and failure analysis.
XPS Spectroscopy Examples
Examples of XPS Analysis include:
- Identifying stains and discolorations
- Characterizing cleaning processes
- Analyzing the composition of powders and debris
- Determining contaminant sources
- Examining polymer functionality before and after processing to identify and quantify surface changes
- Obtaining depth profiles of thin film stacks (both conducting and non-conducting) for matrix level constituents and contaminants (down to the low % level)
- Assessing the differences in oxide thickness between samples
- Measuring lubricant thickness on hard disks
Survey XPS analysis (wide energy range)
Except for H and He, XPS scans the entire energy range to present.
In other words, an application for identification and quantification of elements present on a surface.
High resolution XPS analysis (narrow energy range)
To get more detailed chemical state information, XPS Analysis uses narrow energy ranges that scan under high energy resolution conditions.
Application: Identify chemical state from detailed peak position and peak shape (it is typically necessary to firs confirm which elements are present by doing a survey XPS analysis, for instance.
Depth profile XPS analysis
In this mode narrow energy ranges are scanned. For non-destructive depth profiling of the outer ~10-20 nm is examined by tilting the sample to modify the sampling depth. For destructive depth profiling of depths up to a few microns the sample is first examined with narrow energy ranges and is then ion sputtered to remove the desired amount of material. These two steps are repeated until the final required depth of XPS analysis is achieved.
Application: Measurement of elemental composition with respect to depth. In non-destructive depth profiling, chemical states can be determined. In most destructive depth profiling, chemical state information is not available because changes to the chemistry often occur during sputtering.

XPS Spectroscopy Ideal Uses
- Firstly, surface analysis of organic and inorganic materials, stains, or residues
- Secondly, determining composition and chemical state information from surfaces
- Thirdly, depth profiling for thin film composition
- In addition, thin film oxide thickness measurements (SiO2, Al2O3)
XPS Strengths
- Chemical state identification on surfaces
- Identification of all elements except for H and He
- Quantitative analysis, including chemical state differences between samples
- Applicable for a wide variety of materials, including insulating samples (including paper, plastics, ceramics, and glass)
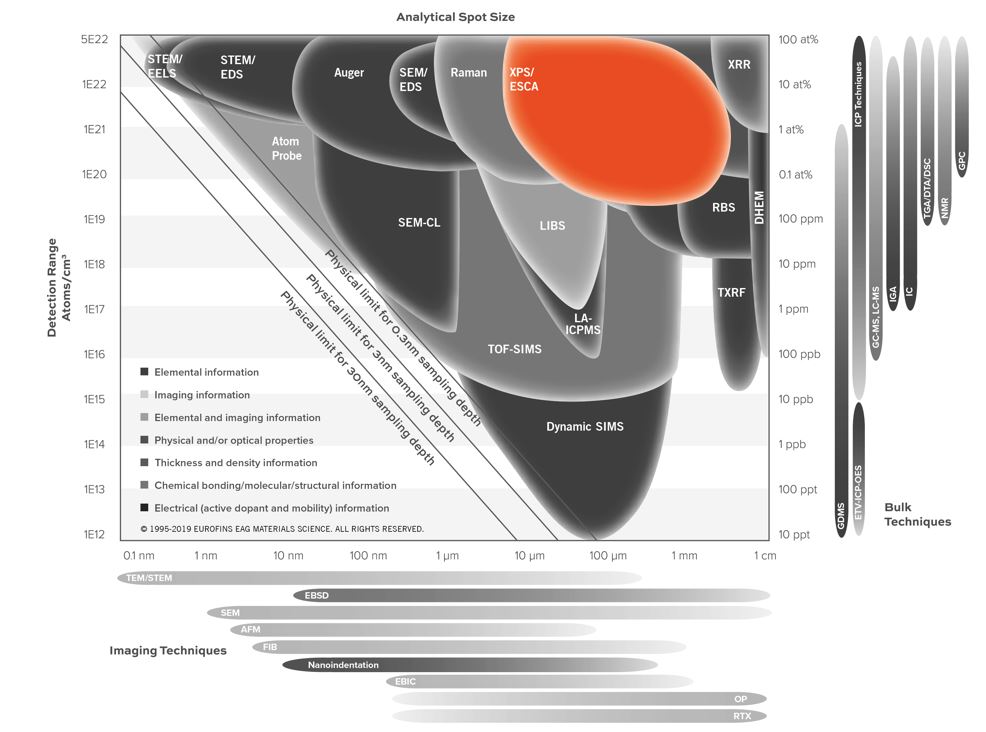
- Surface sensitive (1-10 nm analysis depth)
- Depth profiling for major elements (generally > 1 atomic%)
- Oxide thickness measurements
XPS Limitations
- Detection limits typically ~ 0.1 at%
- Smallest analytical area ~30 µm
- Limited specific organic information (no long-range bonding information)
- Sample must be UHV compatible
XPS Spectroscopy Technical Specifications
- Signal Detected: Photoelectrons from near surface atoms
- Elements Detected: Li-U Chemical bonding information
- Detection Limits: 0.1–1 at% sub-monolayer
- Depth Resolution: 20–200 Å (Profiling Mode); 10–100 Å (Surface analysis)
- Imaging/Mapping: Yes
- Lateral Resolution/Probe Size: 10 µm – 2 mm
Above all, XPS Spectroscopy insights into a sample’s chemical makeup allow you to make product and process improvements more quickly, enabling you to reduce cycle time and save money.
In addition, with EAG, you also have access to the best facilities, instruments and scientists available for performing XPS Spectroscopy analyses. We handle many different materials from multiple industries, giving us a very wide range of experience. Lastly, our person-to-person service ensures that you will receive answers to all of your questions.
Related Resources
- Webinar: XPS
- XPS App Notes
- App Note: Surface of Pharmaceuticals by XPS
- App Note: Delamination of Multi-layer Metal Foil by XPS
- App Note: Contact Lenses: Understanding Surface Chemistry
- App Note: Adhesion Failure in Medical Device Packaging
- App Note: XPS Analysis of Disposable Gloves
- App Note: Surface and Interface Characterization of Polymers
- App Note: Evaluation of Cleaning Efficacy
- App Note: Glass Delamination in Pharmaceutical Vials
Would you like to learn more about using XPS?
Contact us today for your X-ray Photoelectron Spectroscopy (XPS) need. Please complete the form below to have an EAG expert contact you.