Characterization of Bioceramics for Surgical Implants: Part I – Precursor Qualification
Home » Characterization of Bioceramics for Surgical Implants: Part I – Precursor Qualification
Bioceramics are an important subset of biomaterials, employed in medical and orthopedic applications, mainly for the repair and replacement of diseased and damaged parts of the human skeleton, bone, teeth and joints. Based on the common response of tissues to implants, bioceramics are classified into three groups: (1) bioinert ceramics, e.g., Al2O3, and ZrO2; (2) bioactive ceramics, e.g., hydroxyapatite (HAp); and (3) bioresorbable ceramics such as β- tricalcium phosphate (β-TCP).
With the advent of many bioceramic compositions, it has been the primary focus of this field to ensure the safety of newly developed or currently used materials, by proving their biocompatibility and understanding their toxicological profile, at cytotoxic, histotoxic and genotoxic levels.
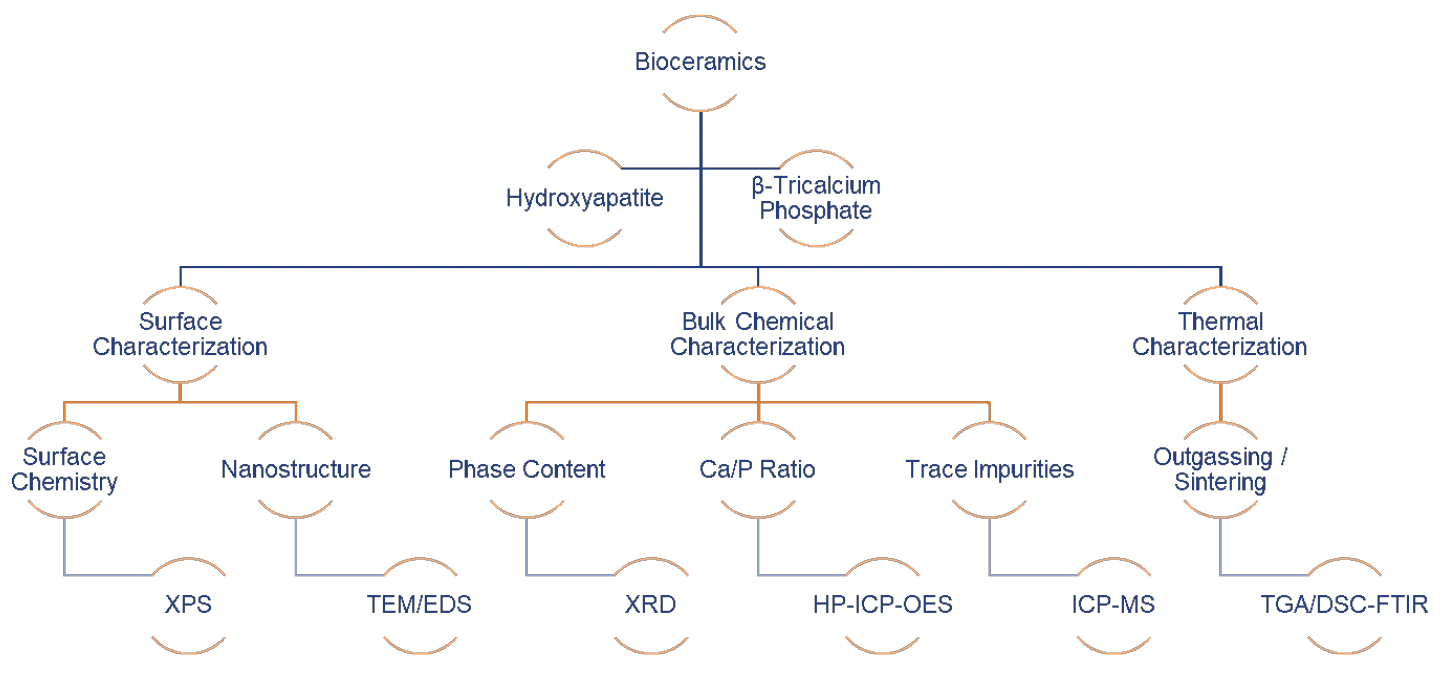
The safety and reliability concerns regarding bioceramics originates from the potential prolonged leaching of ions in the physiological environment, from the integrity of the tissue/implant interface, and from the fatigue fracture and wear behavior of load-bearing implants. HAp, β-TCP, and calcium sulfate hemihydrate and dihydrate have long been recognized as appropriate materials for bones, due to their chemical and biological similarity to human hard tissues. Properly screening bioceramic powders and fabricated implant parts, prior to in vitro and in vivo tests, is appealing in term of safety, and time- and cost-savings. Below are a set of FDA-recognized consensus standards for qualifying these materials for surgical implants:
- ASTM F1185-03 (Reapproved 2014): Standard Specification for Composition of Hydroxyapatite for Surgical Implants
- ASTM F2224-09 (Reapproved 2014): Standard Specification for High Purity Calcium Sulfate Hemihydrate or Dihydrate for Surgical Implants
- ISO 13175-3 1st edition 2012-10-01: Implants for surgery — Calcium phosphates — Part 3: Hydroxyapatite and beta-tricalcium phosphate bone substitutes
- ASTM F1088-18: Standard Specification for Beta-Tricalcium Phosphate for Surgical Implantation
- ISO 13779-2 3rd edition 2018-12: Implants for surgery -Hydroxyapatite – Part 2: Thermally sprayed coatings of hydroxyapatite
- ISO 13779-3 2nd edition 2018-12: Implants for surgery – Hydroxyapatite – Part 3: Chemical analysis and characterization of crystallinity ratio and phase purity
This paper will demonstrate how modern analytical tools (Figure 1) can be used for the quality control of hydroxyapatite and β-tricalcium phosphate powders, which are either directly manufactured into bulk parts after proper sintering, or spray coated on load-bearing implants to promote initial osseointegration. Specifically, we will demonstrate EAG’s capability in terms of chemical, surface and thermal characterization of bioceramic precursors used for surgical implants (Sigma Aldrich® HAp Lot# 289396, unsintered β-TCP Lot# 13204 and sintered β-TCP Lot#49963).
Figure 1 shows the modern analytical technique matrix for characterizing bioceramic precursors: X-Ray Photoelectron Spectroscopy (XPS), Transmission Electron Microscopy/Energy Dispersive X-ray Spectroscopy (TEM/EDS), X-ray Diffraction (XRD), High Performance Inductively Coupled Plasma – Optical Emission Spectroscopy (HP-ICP-OES), Inductively Coupled Plasma – Mass Spectroscopy (ICP-MS), and Thermogravimetric Analysis/Differential Scanning Calorimetry – Fourier Transfer Infrared Spectroscopy (TGA/DSC-FTIR).
Would you like to learn more about Characterization of Bioceramics?
Contact us today for your characterization of bioceramics for surgical implant needs. Please complete the form below to have an EAG expert contact you.