Ask the Expert: Is Your Wearable Device Safe?
Home » Ask the Expert: Is Your Wearable Device Safe?
Wednesday, August 14, 2024
11:00 am PT / 1 pm CT / 2:00 pm ET
Missed our event? Fill out the form below to view a recording of the presentation.
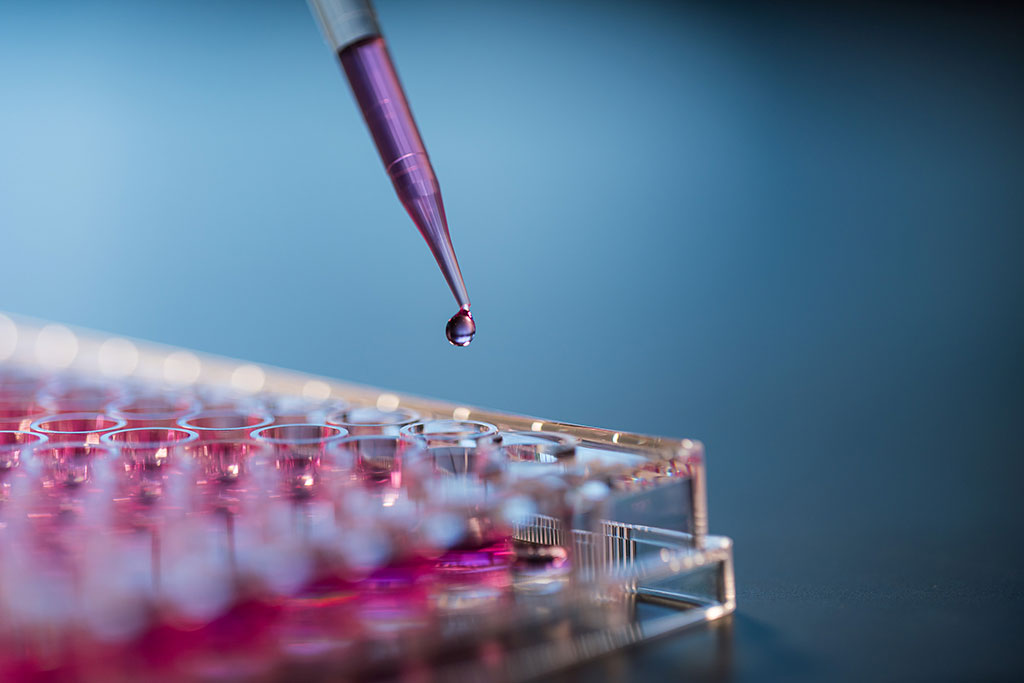
Pioneered for the medical device industry, in vitro biocompatibility testing is a powerful tool in the risk assessment of wearable devices.
Product development of biomaterials – from implants to wearable consumer products – requires a careful balance of risk management and operational constraints. Traditional methods of biocompatibility evaluation, i.e., animal models for toxicology assessment, can be burdensome on timelines, budgets, and the animal population. Alternatively, new in vitro approaches exist to deliver fast, cost effective, and reliable biological data.
Eurofins EAG Laboratories offers a variety of in vitro biocompatibility assays to evaluate biological endpoints such as cytotoxicity, irritation, and sensitization of medical devices and consumer products. In this webinar, we will explore how in vitro assays are used to frequently screen products in development or production to deliver fast answers to help companies make well-informed decisions. By this, companies can maintain control over their supply chains and ensure their devices are safe. From design changes to lot release, in vitro biocompatibility assays add value and offer assurance in taking the next steps in the product lifecycle.
During this live Ask the Expert webinar, we answered pre-submitted questions from our audience regarding in vitro biocompatibility testing for the risk assessment of wearable devices.
Missed our event? Fill out the form below to view a recording of the presentation.
Experts
- Blake Darkow, M.S.
Team Leader - Philip Ferko, Ph.D.
Team Leader & Senior Scientist
