In Vitro Cytotoxicity Testing
Home » Services » Materials Testing & Analysis » In Vitro Cytotoxicity Testing
Customized Cytotoxicity testing for your product
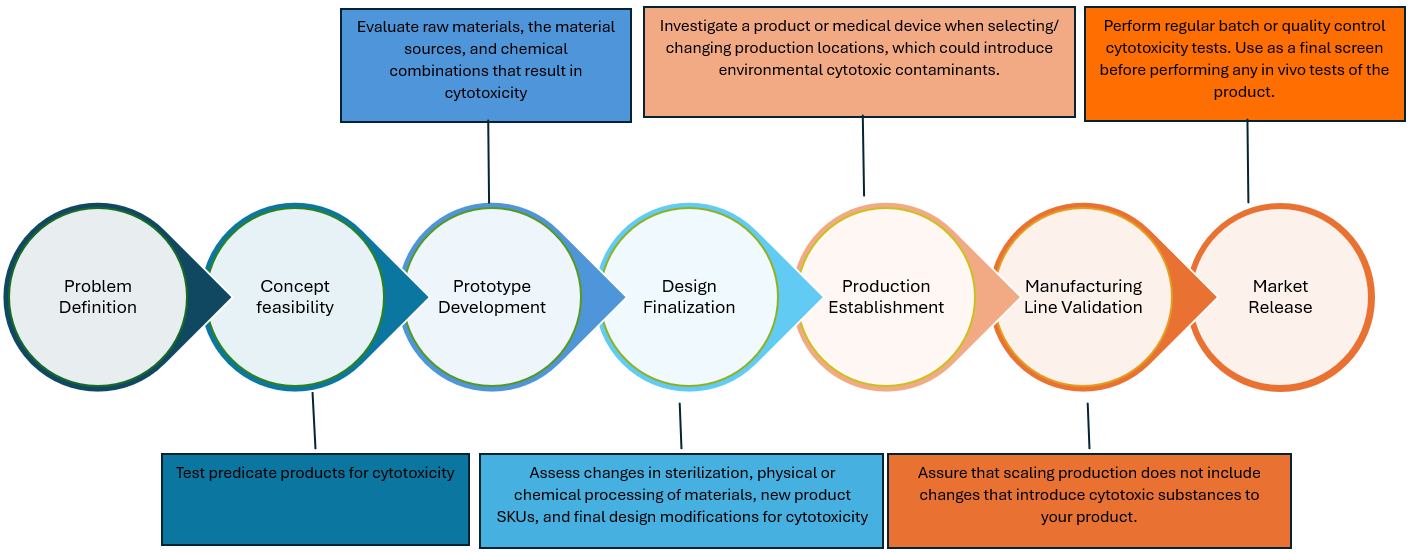
Test a consumer product, medical device, or raw material for cytotoxicity based on its anticipated use and status in the product development process. The test article’s physical characteristics and function are considered to build an experiment that provides an accurate, reliable, and ethical assessment of general toxicity risk. All tests are performed by evaluating the health of mammalian fibroblast cells before and after exposure to the sample with rapid results generated through qualitative or quantitative methods. These results indicate if toxic substances are present on or within the test article that could harm the end user.
A modular approach to Cytotoxicity testing creates experiments designed for your product

Safety Screening
In vitro cytotoxicity testing is an efficient, fiscally favorable and cruelty-free way to screen your sample during conceptualization, development, and manufacturing of your test article. Reliable results with fast turnaround time may be used as a risk mitigation measure that assures quality throughout the product development process and after market release. Early detection of cytotoxicity protects your company and your customers by identifying and eliminating biocompatibility issues that arise. Tests are performed on a culture of healthy fibroblast cells, allowing for fast results to be delivered using a smaller number of samples and without the need for animal testing.
FDA Regulatory Submission
For medical devices, in vitro cytotoxicity tests will be performed by following the methods in ISO 10993-5 and ISO 10993-12, with a detailed report that covers the necessary information for regulatory submission. In vitro tests may also be used preliminarily before proceeding to other endpoint tests such as irritation, sensitization, hemocompatibility, or genotoxicity. Additionally, per the FDA’s emphasis on reducing the need for tests on laboratory animals, the in vitro cytotoxicity tests can be used before any in vivo assessments to identify biocompatibility issues of the product. These preliminary tests allow you to modify the product or identify the cause of toxicity before spending time or finances on in vivo tests.
Learn More About Cytotoxicity Benefits
Resources on what to do after a product is identified as cytotoxic, as well as educational materials on the application and performance of cytotoxicity assays, are available on the Eurofins EAG website. Contact us today for your In Vitro Cytotoxicity testing needs.
Visit our blog to learn more about the principles and strengths of In Vitro Testing.
Would you like to learn more about In Vitro Biocompatibility Testing?
Contact us today for your In Vitro Biocompatibility testing needs. Please complete the form below to have an EAG expert contact you.