Corrosion Testing
Home » Services » Materials Testing & Analysis » Corrosion Testing
The Importance of Understanding the Corrosion of Materials
Corrosion is the deterioration of materials (typically metal) caused by an electrochemical reaction with their environment. Corrosion testing is critical to understanding how a material reacts with its environment. Clients use Eurofins EAG corrosion testing services to solve, prevent, and mitigate corrosion problems including, premature failure, structural integrity, and contamination. In addition, corrosion testing helps ensure proper materials selection, a critical manufacturing step that prevents future problems.

Medical Device Corrosion Testing: Eurofins EAG Laboratories is ISO 17025 Accredited for ASTM F2129
Corrosion testing is particularly important in medical device systems since uncontrolled release of metallic ions can have damaging biological effects.
ASTM F2129 (Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices) is a corrosion susceptibility test for small implant devices, individual components of the devices, or other material samples made from metal. Potentiodynamic polarization in a cyclic manner (forward and reverse) is used to perform the test.
Medical devices which can be assessed by ASTM F2129 include but are not limited to:
- Vascular stents
- Ureteral stents
- Filters
- Support segments of endovascular grafts
- Cardiac occluders
- Aneurysm or ligation clips
- Staples
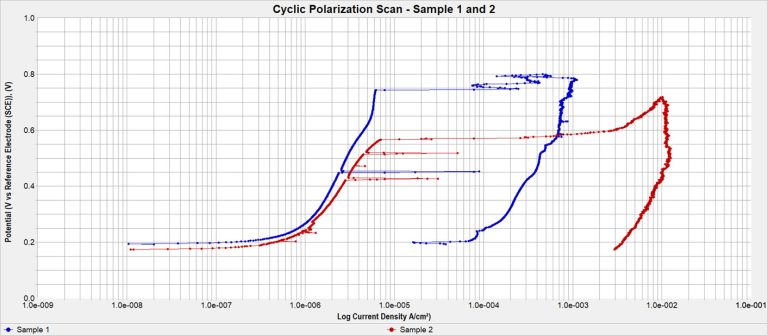
The cyclic potentiodynamic polarization tests are conducted in accordance with the most current version of ASTM F2129. This standard specifies that a specimen be exposed to a specific solution (phosphate buffered saline electrolyte (PBS) or another medium depending on what the medical device is exposed to in the human body) in a test cell with a saturated calomel electrode (SCE) as the reference electrode and a counter electrode (graphite).
The FDA recognizes the complete standard and accepts ASTM F2129 corrosion testing to assess the robustness of the oxide layer of the final finished form of the medical device (in the condition of which it enters and exists in the body).
Eurofins EAG can accommodate any customization needed for ASTM F2129 corrosion testing for internal quality control and third party verifications.
In addition, SEM imaging can be added before and after corrosion testing to further characterize the corrosion.
Corrosion Failure Analysis and Consulting
Eurofins EAG corrosion consultants help clients enhance existing products before and after corrosion testing. Even if testing is not needed, our consultants can provide failure analysis to understand why corrosion mechanisms caused the failure and consulting for prevention.

Additional ASTM and ISO Corrosion Testing Available
- ASTM G69 Standard Test Method for Measurement of Corrosion Potentials of Aluminum Alloys
- ASTM F3044 Standard Test Method for Evaluating the Potential for Galvanic Corrosion for Medical Implants
- ASTM G5 Standard Test Method for Making Potentiodynamic Anodic Polarization Measurements
- ASTM F746 Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials
- ASTM D1654 Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to the Corrosive Environments
- ASTM D610 Standard Practice for Evaluating Degree of Rusting on Painted Steel Surfaces
- ISO 10555-1 Intravascular catheters – Sterile and single-use catheters – Part 1: General Requirements Annex A – Test method for corrosion resistance
- ASTM G31-21/NACE TM0169 Standard Guide for Laboratory Immersion Corrosion Testing of Metals
- ASTM G1-03 Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens
Customization for any of the above corrosion testing is available upon request.
Would you like to learn more about Corrosion Testing?
Contact us today for your Corrosion Testing needs. Please complete the form below to have an EAG expert contact you.