Determination of Flame-retardant Materials in Plastics
Home » Determination of Flame-retardant Materials
Flame retardant compounds serve an important purpose in society and are particularly critical in plastics, which are often more flammable than other materials. To evaluate the efficacy of flame retardants in commercial products, it is important to know both the concentration and composition. However, due to the variability of flame retardants, the appropriate analytical method is not always obvious. In this publication, we analyze an unknown plastic box advertised to have flame retardant properties. We use a series of analytical techniques and evaluate their compatibility with one another.
INTRODUCTION
Flame retardants materials constitute an important class of materials for protecting life and property. These compounds can help slow or stop the spread of fire, and as such are typically found in a variety of consumer goods.

The exact composition and concentration of these compounds is essential for determining their efficacy, especially in cases of litigation.1
Many different retardant materials are commercially available, including brominated, phosphorus, nitrogen, chlorinated, and inorganic compounds such as hydrated aluminum, magnesium oxide, and antimony trioxide.2-4
Due to the variety of flame retardant materials, several analytical tools must be employed to determine the composition. In this publication, a commercially available plastic box was analyzed using a combination of fourier transform infrared spectroscopy (FTIR), x-ray fluoresence spectoscopy (XRF) and gas chromatography mass spectroscopy (GCMS) to illustrate the power of complimentary techniques to determine the composition and concentration of the flame retardant component.
EXPERIMENTAL
A plastic box shown in Figure 1 was purchased from an online retailer. The box was cut into three pieces using a band saw. Experiments were performed using FTIR, XRF and GCMS techniques as described below.
FTIR: A small portion of the sample was transferred to an infrared transmitting substrate and examined using a Thermo- Nicolet 6700 Fourier Transform Infrared (FTIR) spectrometer equipped with a Continuum microscope in transmission mode. The analytical spot size was approximately 100 microns x 100 microns. OMNIC 8.0 software was used to perform data analysis.
XRF: X-ray Fluorescence (XRF) was performed using a Rigaku Primus II WDXRF with a rhodium X-ray source x-ray tube, vacuum atmosphere and an analysis area of 30 mm diameter. This analysis utilized a wavelength dispersive spectrometer (WDXRF) that is capable of detecting elements from atomic number (Z) of 4 (beryllium) through atomic number 92 (uranium) at concentrations from the low parts per million (ppm) range up to 100% by weight.
GCMS: An extract was prepared by dissolving a small piece of plastic in dichloromethane. The extract was analyzed on an Agilent 6890A Gas Chromatograph/ Agilent 5973 Mass Spectrometer with a mass selective detector (MSD) using a 30 m X 0.25 mm DB-5MS (J&W Scientific) column under a flow rate of 1 mL/min. Once the flame retardant material was determined, a 1-point calibration curve was prepared using a commercial standard with a known concentration.
RESULTS AND DISCUSSION
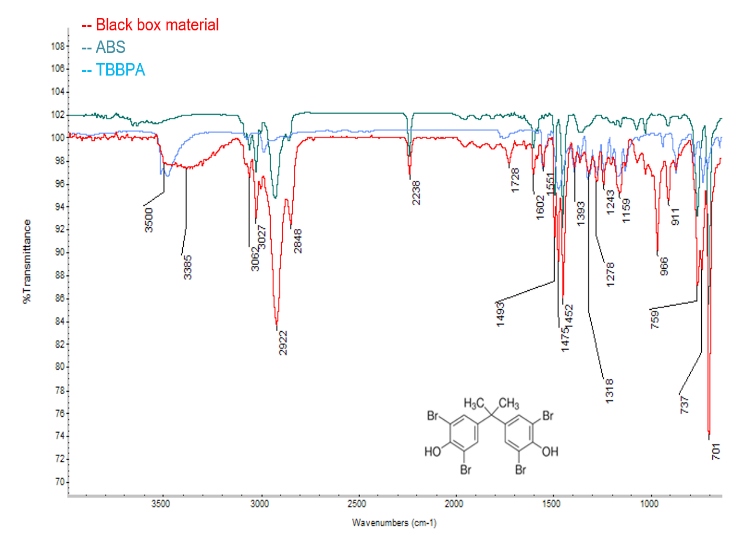
A commercially available plastic box shown in Figure 1 was examined by FTIR in transmission mode.
The resulting spectrum is shown in Figure 2 (black box). The spectrum is dominated by peaks at 3062, 3027, 2922, 2848, 2238, 1602, 1475, 1452, 759, and 701 cm-1, which are due to acrylonitrile butadiene styrene (ABS) as determined by comparison with library references.
In addition to ABS, several other peaks were observed at 1551, 1475, 1393, 1318, 278, 1243, 1159, and 737 cm-1, which are due to tetrabromobisphenol A (TBBPA). TBBPA is a commonly used fire retardant in a variety of applications.3,4 It is also an initiator polymerization of ABS. Furthermore, a trace ester is observed at 1728 cm-1, which may be an additive in the plastic. Altogether, these results demonstrate that TBBPA is the main flame retardant component in the plastic and that the base polymer is ABS.
XRF was performed directly on a small piece of plastic and the results are summarized in Table 1.
a The results are normalized to 100% of the measured and detected elements
b At levels observed, the concentrations may have a considerable contribution from instrumental background
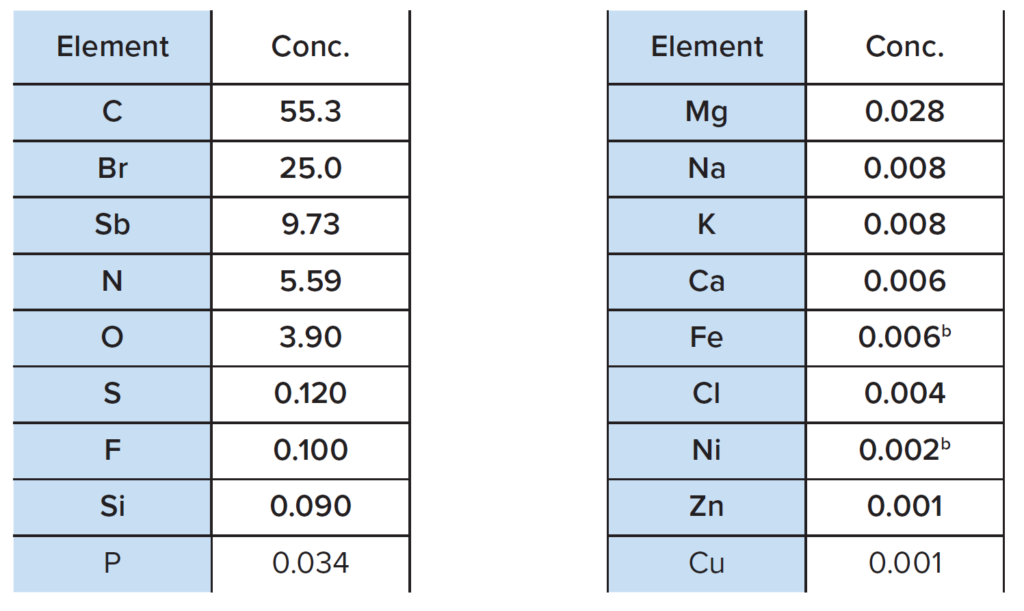
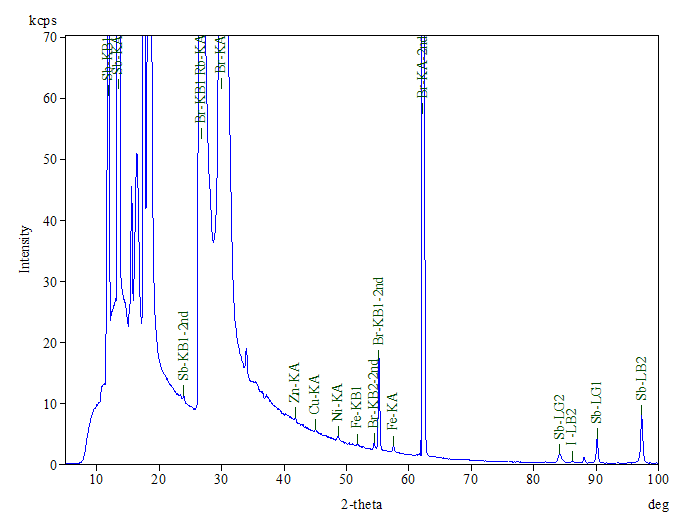
A representative XRF spectrum is shown in Figure 3. The main elements detected include C, Br, Sb, N and O.
The presence of C and N confirms the FTIR finding of ABS base polymer, while the high bromine level supports the presence of TBBPA as a flame retardant component. The ~10% antimony content also suggests that antimony trioxide may be a second flame retardant component in the plastic, which is also supported by the ratio of Sb to O.
Antimony trioxide is an inorganic flame retardant commonly used in combination with brominated compounds.5
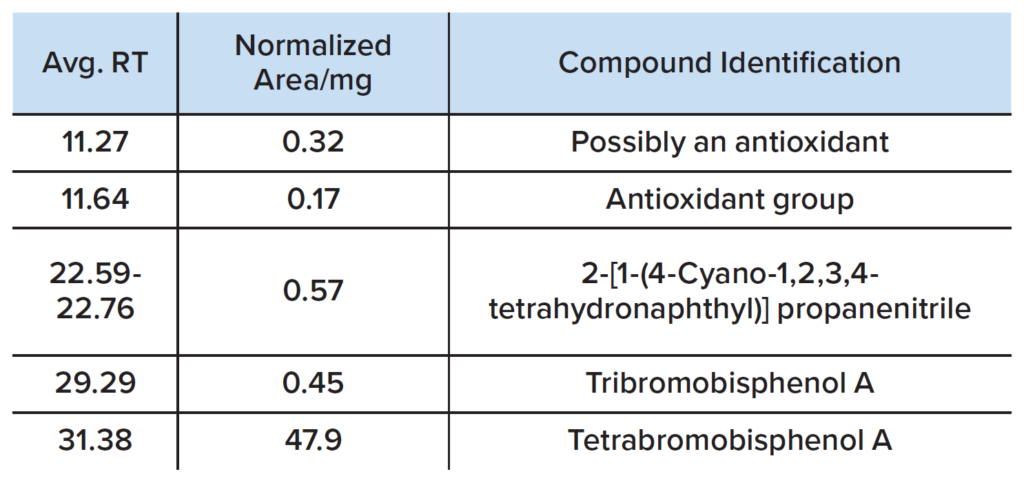
GCMS analysis was performed on a dichloromethane polymer extract and the major volatiles are summarized in Table 2.
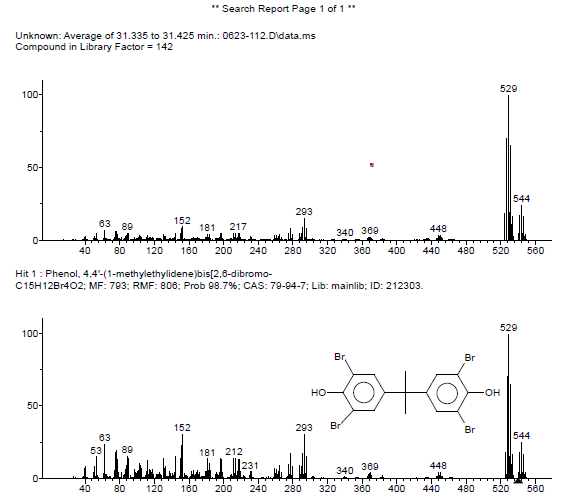
The presence of TBBPA is again confirmed by the mass spectrum shown in Figure 4. Other compounds are observed in the plastic, including antioxidant(s) and ABS-derivatives. Note that tribromobisphenol A is a de-bromination product of TBBPA. 6
CONCLUSIONS
A plastic box containing an unknown flame retardant was examined using a suite of analytical techniques including FTIR, XRF and GCMS. The flame retardant components were identified as tetrabromobisphenol A (TBBPA) and possibly antimony trioxide. The amount of TBBPA was determined to be ~25 wt% by XRF and ~28% by GCMS. The proximity of the two values demonstrates the compatibility of the methods. Altogether, the results suggest that the combination of techniques produces complimentary and reliable information about the flame retardant composition.
REFERENCES
- E. D. Weil and S. Levchik, Journal of Fire Sciences 22, 25 (2004).
- S. Zhang and A.R. Horrocks, Progress in Polymer Science. 28, 1517 (2003).
- L. Chen and Y.-Z Wang. Polym. Adv. Technol., 21 1–26 (2010).
- E. D. Weil, Journal of Fire Sciences 29, 259 (2011).
- U. Sellström, B. Jansson, Chemosphere, 31, 3085 (1995).
- J. Eriksson, S. Rahm, N. Green, Å. Bergman and E. Jakobsson. Chemosphere, 54, 117 (2004).
Would you like to learn more about Determination of Flame-retardant Materials?
Contact us today for your determination of flame-retardant materials in plastic needs. Please complete the form below to have an EAG expert contact you.