Residual Solvents in Medical Devices
Home » Residual Solvents in Medical Devices
Levels of residual solvent concentrations in medical devices such as polymer coatings on stents are restricted by the US Food and Drug Administration.
Dynamic headspace Gas Chromatography-Mass Spectromety (GC-MS) is an extremely useful way to measure residual solvents because it requires no liquid extraction or other preparation. The technique also has very good sensitivity and repeatability. Together, these features make GC-MS the preferred method to measure residual solvents.
Dynamic headspace works by subjecting the sample to elevated temperatures for a period of time, driving volatile compounds from the sample matrix into the atmosphere above the sample.
The outgassed volatiles are collected on a cooled trap and then separated into individual components by the GC. Components eluted from the GC column are identified in the mass spectrometer. The concentrations of the residual solvents are determined using known weight samples and calibration curves prepared using pure solvents.
The following figures and spectra give an example of the use of GC-MS to detect and measure residual solvents.
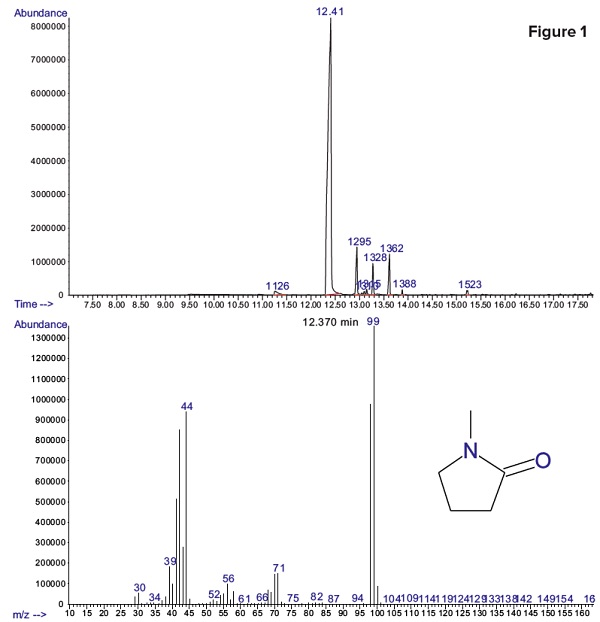
Figure 1 shows that residual methyl-pyrrolidone (NMP) was detected with a peak retention time of 12.41 minutes. Dimethyl sulfoxide (DMSO) was also possibly present in the sample but no peak indicating residual DMSO was detected.
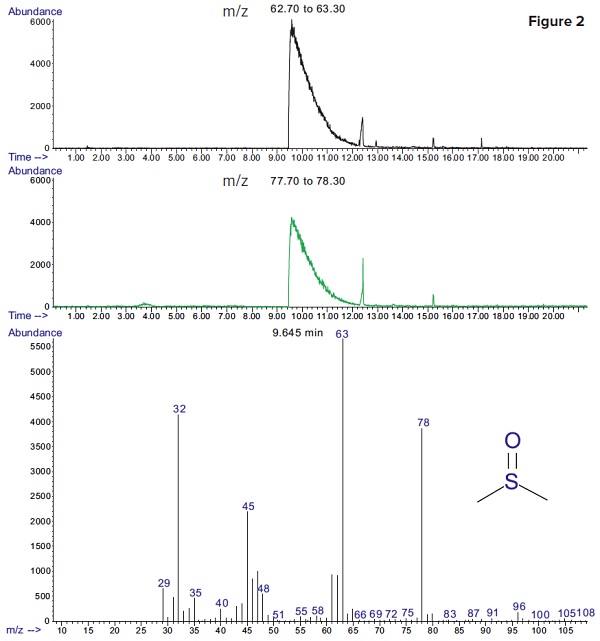
Figure 2 shows extracted ion chromatograms of major fragments of DMSO (m/z=63 and 78). DMSO was detected with a much better sensitivity, using selective ion monitoring.
Would you like to learn more about Residual Solvents in Medical Devices?
Contact us today for your residual solvents in medical device needs. Please complete the form below to have an EAG expert contact you.