Quantification of Active Pharmaceutical Ingredients by XPS
Home » Quantification of Active Pharmaceutical Ingredients by XPS
There is an interest in controlling and understanding the concentration of active pharmaceutical ingredients (APIs) and excipients on pharmaceutical powder surfaces for a host of reasons. Some of this interest is driven by decreasing particle size for aerosols or nanomedicine applications where the atoms in the near-surface make up a significant fraction of the total atoms as the size decreases into the sub-micron range. In addition to concerns based purely on size, the outer surface of pharmaceutical particles is often chemically quite different from the bulk. Processing can result in the segregation or coating of one component relative to others at the surface of a particle. This, in turn, affects a host of performance parameters including solubility, dissolution rates, stability, flowability, agglomeration and crystallinity. With its surface sensitivity, standardless quantification and ability to determine the nearest neighbor chemical bonds, X-ray Photoelectron Spectroscopy (XPS) is uniquely positioned to analyze the surface chemistry of pharmaceuticals.
DISCUSSION
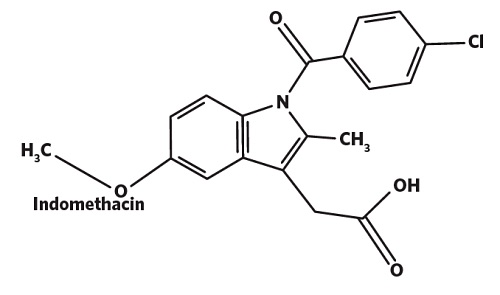
In this example, XPS is used to quantify the amount of a COX inhibitor (Indomethacin, C19H16ClNO4) spray dried with a triblock co-polymer of polyethylene glycol –polypropylene glycol- polyethylene glycol (Poloxamer 407) and sodium carboxymethyl cellulose (CMC).
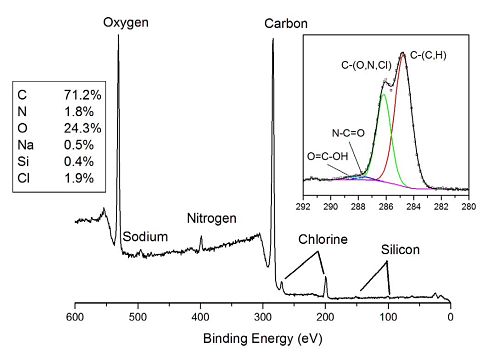
The photoelectron spectrum shows distinct peaks for the elements expected in the sample (C, O, N, Cl and Na) as well as minor amount of Si from an unknown source. The inset shows the high energy resolution carbon spectrum. Four different bands are detected in carbon spectrum. The lowest binding energy band (284.8 eV) is from –CH3 and carbon in the aromatic ring structure of indomethacin. The large peak at 286.3 eV is due to C-O, C-Cl and C-N bonds present in the API and/or the excipients. The weak peak at ~287.7 eV is due to N-C=O and the other weak peak at ~288.5 eV is due to COOH.
The % indomethacin is determined from the measured amount of N (or Cl) that only comes from that material. Sodium is used to quantify the amount of CMC. The Poloxamer 407 is determined by difference. The atom fraction of indomethacin on the surface of this sample was 45%. The target (bulk) loading of API in the formulation was 25% confirming a high degree of surface coverage of the drug on the polymer.
There was also interest in checking the homogeneity of the pharmaceutical product. The figure below shows an X-ray induced electron micrograph acquired in the XPS. The image is formed much like in a scanning electron microscope where a focused electron beam is rastered across a sample surface while secondary electrons are collected.
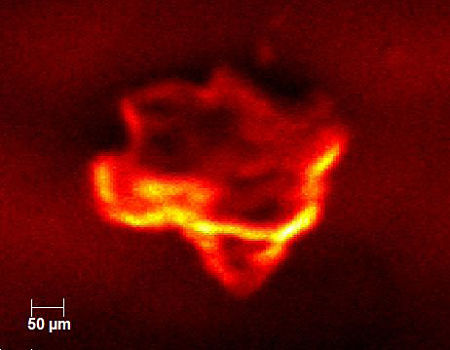
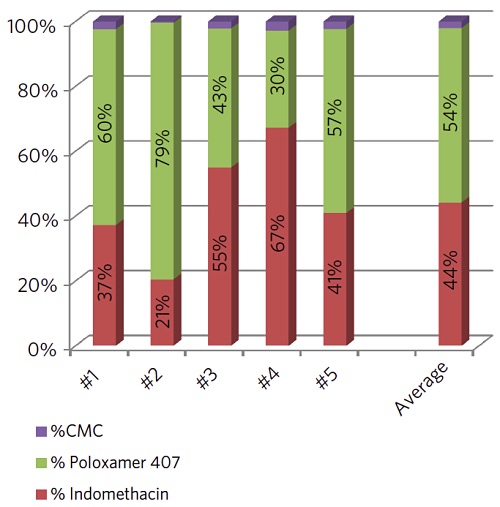
In this case we raster a 5µm X-ray beam across the surface while collecting secondary electrons. The results from five individual particles are summarized in the chart below. The drug concentration varied by more than 3x among the particles analyzed.
However, the average indomethacin concentration detected on the five particles (44%) agreed well with the amount found on the large area analysis of several dozen particles (45%).
We use XPS to quantify the amount of an API (indomethacin) and two excipients (sodium carboxymethylcellulose and Poloxamer 407) present on the outer surface of individual particles. Analyses were done both in aggregate, to provide a snapshot of the average surface composition, as well as on individual particles- to assess surface chemical uniformity.
Understanding the surface concentration of various components in pharmaceutical powders is critical for controlling performance parameters such as solubility, dissolution, stability, flowability, agglomeration and crystallinity.
Would you like to learn more about Quantification of Pharmaceutical Ingredients?
Contact us today for your quantification of active pharmaceutical ingredients. Please complete the form below to have an EAG expert contact you.