Characterization of Polymers using Differential Scanning Calorimetry (DSC)
Home » Characterization of Polymers using Differential Scanning Calorimetry (DSC)
Knowledge of the thermal properties of polymers is essential for developing the best methods for processing the materials into useful products and predicting performance during product lifetimes. It also provides essential information for troubleshooting when the material does not perform as expected, or when something in the product or process needs to be changed, such as raw materials.
IMPORTANCE OF CHARACTERIZING THERMAL BEHAVIORS OF POLYMERS
One of the tools proven to address these needs is differential scanning calorimetry (DSC). This tool features many powerful techniques for studying polymer thermal properties and provides essential information to the polymer industry and end users of polymer-based products. This paper will provide an overview of DSC.
Case studies will be discussed to demonstrate many of the capabilities of this sensitive analytical instrument.
Listed below are some important instances in which thermal analysis addresses specific needs:
- Identifying unknown polymer
- Determining best processing temperatures (cure, injection molding, extrusion, heat welding)
- Comparing quality (failure analysis, new material evaluation)
- Monitoring effects of aging
- Determining phase separation (polymer blend, copolymer)
- Estimating percent crystallinity
- Measuring heat capacity
- Determining thermal stability (oxidation induction time)
- Determining effects of additives (blends, fillers, plasticizers, process aids)
- Measuring residual cure and Tg as a function of cure temperature/time
- Estimating degree of cure
- Setting specifications (verify material meets expectations, set limits for end-use conditions)
- Designing devices (evaluating performance under operating temperatures, choosing materials for specific applications)
- Estimating upper use temperature from Tg or melting point
- Analyzing cure or crystallization kinetics
BASICS OF DSC ANALYSIS
A heat flux DSC (i.e. containing a single heating block) is comprised of a thermal cell with a sensor that registers the temperature difference between the sample-filled pan and a similar reference pan that contains only air (i.e. “empty”). When the sample evolves heat through some thermal process, such as a crosslinking reaction, the DSC plot shows an increase in heat flow. This is indicative of an exothermic event because the temperature registered by the sample sensor is higher than that sensed for the reference. If the sample is undergoing a thermal event that causes it to absorb more heat than the reference does (such as melting), the DSC plot shows a decrease in heat flow.

This is called an endotherm and, in these cases, the temperature sensor measures a lower temperature for the sample compared to the reference. For example, a material can be heated at a controlled steady rate, such as 10°C per minute, and the heat flow can be monitored to characterize the thermal events of the sample as a function of increasing temperature.
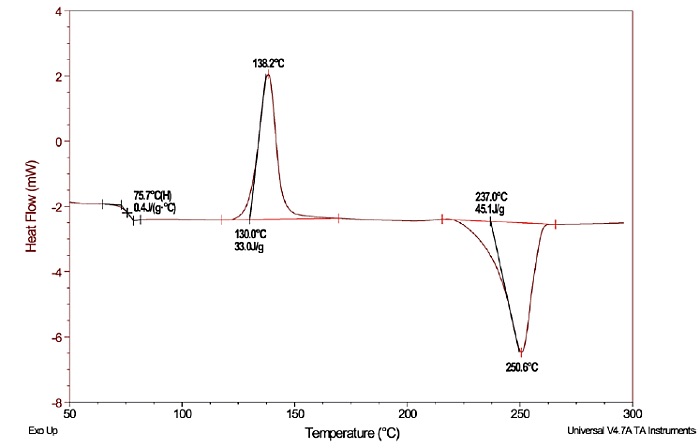
Figure 1 shows a DSC plot for a polyethylene terephthalate (PET) sample, which had been cooled from the melt at an extremely high rate. The plot illustrates both exothermic and endothermic thermal events that occurred during a temperature scan from 50°C to 300°C. The endothermic step change (glass transition) occurs first in the scan, followed by an exothermic peak due to “cold” crystallization, which is then followed by the endothermic peak due to melting.
Many modern DSC instruments have the ability to measure the absolute heat flow. This is done by dividing the signal by the measured heating rate, which converts it into a heat capacity signal. Monitoring the heat-capacity related signal as a function of the applied experimental conditions (such as a heating ramp) can determine how the heat capacity of the sample changes as it undergoes a phase change or a chemical reaction.
Actually, the direct measurement of heat capacity by DSC involves thermodynamic calculations that are built into the instrument and necessitates a few extra calibrations by the operator. The quantity of heat flowing into and out of the DSC sensor is not only dependent on the applied temperature and the properties of the sample, but also on the thermal resistance and capacitance of both sides of the DSC cell (the reference and the sample sides). The mathematical model for how these cell parameters are measured and applied is beyond the scope of this paper. However, a DSC equipped with this technology, trademarked as Tzero™ by TA Instruments Inc., has proven to be a very powerful tool for accurately measuring the heat capacity of organic compounds and polymers.
The baseline, which is the heat flow signal of the DSC in the absence of a thermal event, is much flatter and more reproducible using Tzero Technology. This provides for the identification of weak thermal events and improves the accuracy of heat capacity measurements.
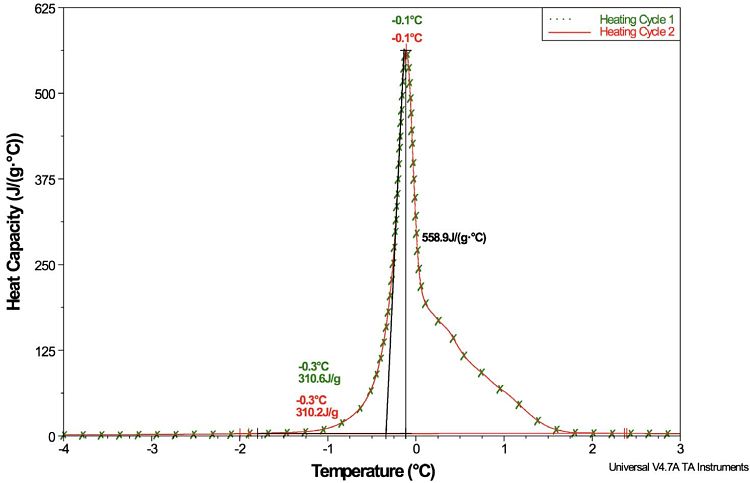
For example, Figure 2 shows a DSC plot of the heat capacity of a phase change material (PCM) that was measured directly during a temperature scan. The heat capacity (Cp) of the PCM increased by ~559 J/(g⋅°C) during the melt transition, which began at approx. -0.3°C. The peak area represents the heat of fusion per gram of material for the PCM. The plot also shows that this phenomenon is reproducible.
Once the PCM has been cooled below the freezing point, and is reheated, the magnitude of the Cp increase is identical.
ADVANCED TECHNIQUES: MODULATED TEMPERATURE DSC
Modulated temperature DSC is a special technique that can offer increased resolution to the measurement capabilities of DSC. This technique, termed MTDSC or MDSC, applies a sinusoidal temperature modulation superimposed over a linear heating rate. During an MDSC experiment, the instrument performs mathematical procedures that separate the heat flow (total) into two different components: (1) reversing signal, and, (2) non-reversing signal.
Properties that respond to the cyclic heating rate are separated into the reversing signal, which include polymer transitions that significantly affect molecular mobility. For example, when a polymer in the glassy phase is heated to a certain temperature, it can undergo a phase change which induces liquid-like flow. This glass transition increases molecular mobility, as well as heat capacity, which determine processability in molding and extrusion operations. For a given polymer, the glass transition temperature (Tg) and the change in heat capacity (ΔCp) can be obtained from the same MDSC experiment. To accomplish this, the MDSC signals are plotted two ways: (1) Reversing Heat Flow versus Temperature for analyzing Tg, and, (2) Reversing Heat Capacity versus Temperature to analyze the change in Cp.
Properties that do not respond to the temperature modulation are those that involve time-dependent (kinetic) transitions, which include crystallization, decomposition, evaporation and chemical reactions (including curing). The heat flow properties associated with these thermal events are found in the MDSC plots of the non-reversing signal. The ability of the MDSC to separate overlapping events, such as Tg and curing, into two distinct plots makes this a very powerful tool for analyzing complex materials and mixtures containing multiple components.
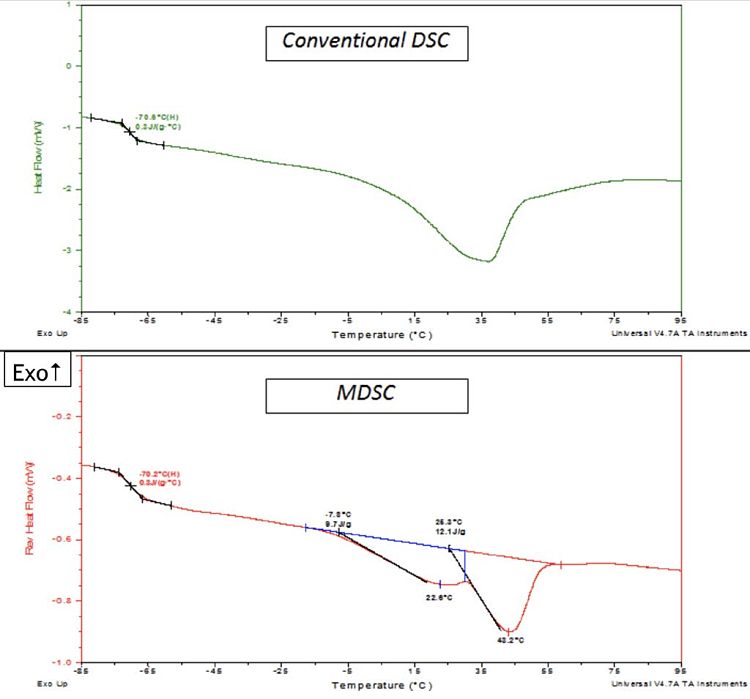
An illustration of the higher resolution that can be achieved using MDSC is presented in Figure 3. The upper portion of the Figure shows conventional DSC (non-modulated) data for a petrolatum sample (scanned at 10°C/minute), and the lower part shows MDSC data obtained for the same sample (scanned at 3°C/minute). Both plots show the same Tg step-change at approx. -70°C. However, the melting peak (above the Tg) shows dissimilarities between the two different techniques. The conventional DSC plot does not have a well-defined peak shape. This makes it difficult to pinpoint the exact location of the peak maximum, as well as the start and end points, which define the melting point and the melting range, respectively. By comparison, the MDSC data reveals the complexity of the melting behavior of the petrolatum which can be transformed into meaningful information. For example, the reversing heat flow plot suggests two overlapping melting peaks, which can be integrated to provide melting points of the two suspected types of crystalline fractions in the material.
Long term storage of polymers below Tg typically results in a gradual process of molecular relaxation. Relaxation is a phenomenon that occurs when amorphous polymer chains form abnormally denser regions.
This densification can adversely affect performance by causing embrittlement, dimensional change and/or the development of internal stresses. DSC is a useful tool for studying this phenomenon known as “physical aging”.
The initial DSC heating scan of physically aged polymers commonly reveals an endothermic peak near the trailing edge of the Tg step-change. This peak is referred to as the “enthalpy of relaxation” or “enthalpic recovery” (ΔHR). Heating to Tg allows polymer chains move to a more relaxed condition; that is, the chains spring back to normal (pre-aging) volume and density conditions. The ΔHR transition corresponds with heat flow associated with this movement, and the magnitude of the peak is a measure of the extent of aging. When the analysis is conducted using conventional DSC, the Tg and ΔHR overlap. The “Conventional” DSC plot in Figure 4 shows the Tg of plasticized polyvinyl chloride (PVC) plus the ΔHR peak, which illustrate this transition overlap.
The MDSC data in Figure 5 demonstrates the effective separation of these transitions: Tg is separated into the reversing heat flow plot, and the ΔHR is separated into the non-reversing heat flow plot. As shown, the ΔHR peak can even be integrated to calculate the magnitude of physical aging in the sample.
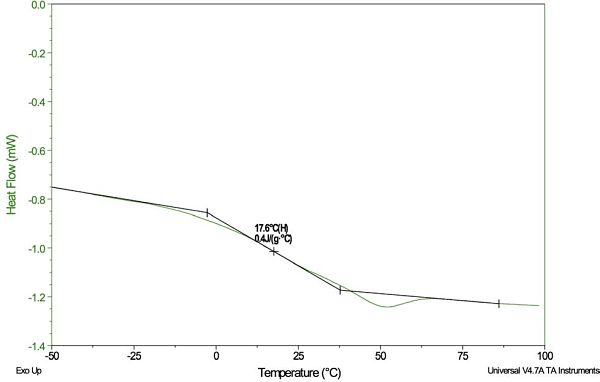
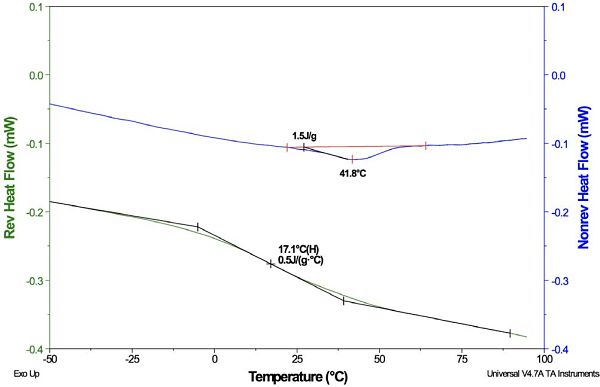
Another powerful MDSC technique has been developed for performing highly accurate heat capacity measurements. This method uses quasi-isothermal conditions, which expose the sample to a temperature modulation that is cycled around a single temperature. If the sample is held long enough at this particular temperature, and the sample does not undergo any transitions, the plot of Cp versus time will appear linear (flat) at some given time. The “end point” of this steady-state condition is considered to be the heat capacity of the sample at that particular temperature.
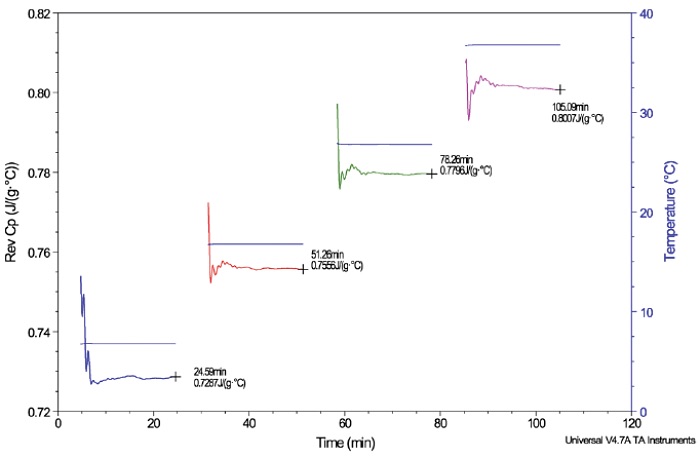
Figure 6 shows a plot of the Cp of aluminum oxide at the temperatures 6°C, 16°C, 26°C and 36°C, labeled with the steady-state “end point” Cp values. The aluminum oxide experiment is used to verify DSC accuracy by comparing the Cp data with the literature values at the corresponding temperatures. After verification, the polymer samples of interest can be run using a series of temperatures within the verified performance range.
Quasi-isothermal DSC (QiDSC) can also be used to monitor isothermal cure of thermosetting polymers, such as epoxy resins. In this case, the heat capacity of the epoxy decreases as it changes from an uncured liquid to a solid network. Monitoring the Cp signal over time will show that the initial drop in the curve reaches a plateau, which marks the stage when the crosslinking reaction rate has significantly slowed. The most important information that can be determined from the data is the time it takes the curing network to reach the “vitrification” point, which can be calculated from the midpoint of the Cp step change. This represents the time at a given cure temperature that it takes for the Tg of the network to reach the applied curing temperature. Beyond this time point the reaction will become sluggish, which can necessitate extra processing time or post-baking that will reduce throughput of cured parts. This information is useful when comparing a series of different isothermal curing temperatures to narrow down the best curing conditions for achieving maximum cure with the highest throughput.
CASE STUDIES FOR DSC ANALYSIS
The following case studies illustrate the effective use of DSC to solve some real-world problems and challenges involving polymeric materials.
CASE STUDY I: IDENTIFICATION OF UNKNOWN POLYMER USING DSC
Identification of an unknown polymer is often needed, such as when plastic parts have been purchased from a supply chain not directly affiliated with the manufacturer. Normally, identification tasks are outsourced to a lab equipped with an infrared spectrophotometer (FT-IR). Though FT-IR data can reveal the chemical “family” of the material, the identification of the subclass of the polymer often calls for additional analytical methods. One useful and rapid method is to run a DSC temperature scan of the polymer to determine the thermal transitions.
An example case in which DSC pinpointed the polymer subclass involved a molded part designed for use in hot humid conditions. Initially, FT-IR analysis had shown the plastic to be consistent with a polyamide (nylon). However, there are several different types of polyamides commercially available, and the IR information did not reveal which one was in the part. DSC testing was performed on a small slice taken from the part.
The DSC plot, shown in Figure 7, revealed a small glass transition temperature (Tg) at 188°C. The following evidence led to the most likely assignment of the main type of polyamide in the plastic part:
1) The unknown plastic did not show a crystalline melting peak. This ruled out the possibility that the unknown base polymer was one of the commercial aliphatic polyamides (such as nylon 6), which are semi-crystalline materials.
2) The Tg was consistent with a material that has a completely amorphous (non-crystalline) structure. Several commercial semi-aromatic polyamides, which contain high levels of benzene rings, are known to be wholly amorphous.
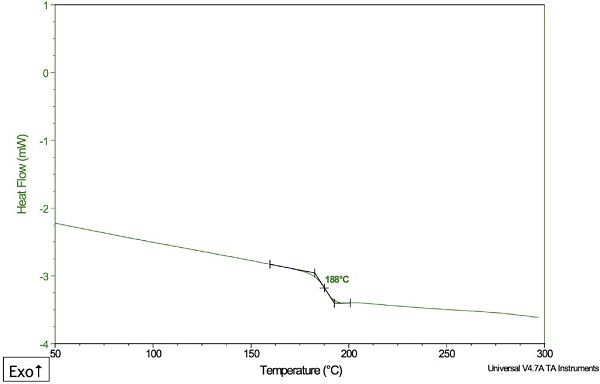
The evidence suggested that the primary polymer composition of the unknown material was a semi-aromatic polyamide, but the Tg of 188°C was significantly above most commercial grades. This information narrowed down the classification of the unknown to a possible blend of semi-aromatic polyamide with a reinforcing agent, such as fiberglass.
CASE STUDY II: THERMAL AND MORPHOLOGICAL VARIATIONS OF DIFFERENT LOTS OF MATERIAL
Variations in thermal and morphological properties can affect polymer performance and processability. These properties can vary among different lots of polymer, despite selection of the best available materials. Worldwide changes in industrial polymer production often make it necessary to find and qualify a new source of polymer material. DSC is an ideal method for investigating the quality of new stocks of material and for comparing lot-to-lot variations in the following properties: Tg (softening/flow), melting, crystallization and percent crystallinity.
An example DSC case study compared two different lots of poly(tetrafluoroethylene) (“PTFE”), which is a semi-crystalline polymer. Before testing, each lot was thermally treated under identical conditions to impart the same thermal history to the crystalline phase.
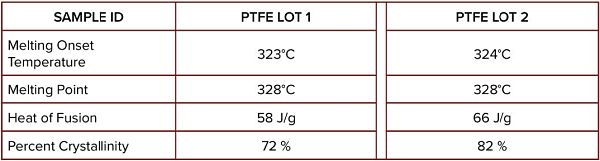
Table 1 compares the melting point data and the heat of fusion (calculated from the area of the melting peak). The melting properties are essentially the same for both lots. However, the heat of fusion values show a marked difference.
The percent crystallinity of each lot was determined by normalizing the measured heat of fusion to the literature value of 100% crystalline PTFE.
As shown, PTFE Lot 1 was 72% crystalline, which was significantly lower compared to the 82% of Lot 2. The lower crystallinity of Lot 1 could probably decrease the material density. Therefore PTFE Lot 1 might not exhibit equivalent performance to Lot 2 since higher crystallinity and higher density provide more advantages such as low moisture permeability and higher mechanical strength.
CASE STUDY III: POLYMER CONTAMINANT INVESTIGATION BY DSC
Cross contamination of a resin with another polymer can occur in melt processing equipment. For example, if extrusion equipment is not purged sufficiently between runs, some residual polymer can be carried over into the new batch of polymer being processed. The appearance of the mixture may still meet expectations, but the products made from the contaminated resin may fail QC testing or fail in the field of use. DSC is a good tool for identifying the presence of contaminating polymeric material when the thermal properties of the contaminant and the base resin are significantly different. The control raw materials suspected to be the contaminant can also be analyzed by DSC.
Using this method, the contaminating resin can be identified by comparing the control data with the data of the “failed” batch.
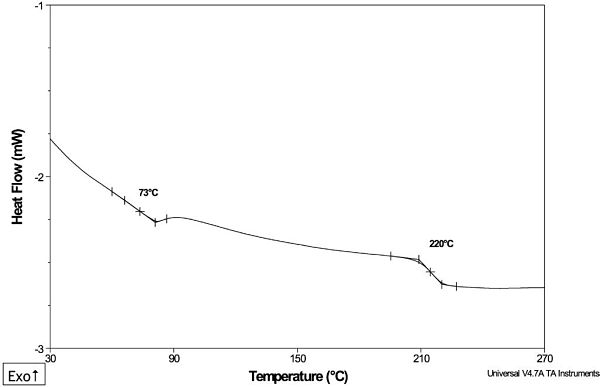
An example case involved a part manufactured from poly(arylether sulfone) (“PAES”) resin. This part showed low hydrolytic stability, resulting in deformation when used under hot, humid conditions.
DSC analysis of a small piece of the material showed the presence of two thermal transitions (Figure 8). The transition at 220°C was the Tg of PAES; the transition at 73°C provided evidence for the presence of a polymeric contaminant. Previous analysis by infrared spectroscopy (IR) had not detected this contaminant because the spectral signals of the PAES resin were predominant.
CONCLUSION
The above cases represent just a few instances where DSC provided relatively quick results which answered fundamental questions and concerns about polymeric materials. Not only were the results obtainable in a timely manner, DSC analysis did not require large samples, numerous controls or extensive method development. These are just a few of the advantages offered by this powerful analytical tool, which has been proven to afford much more measurement capability than determining a melting point.
Would you like to learn more about Characterization of Polymers using DSC?
Contact us today for your characterization of polymers using DSC needs. Please complete the form below to have an EAG expert contact you.