Will the Newest Wearable Device Leave You Itching for More?
Home » Will the Newest Wearable Device Leave You Itching for More?
Wearables are electronic devices that can be worn on the body as an accessory. Examples of consumer wearables include smart watches, smart clothing and smart glasses. In addition to the base polymer, these products contain various additives such as plasticizers, UV stabilizers, pigments or antimicrobial agents. These additives are what give the products their flexibility, color, texture and other properties that may be critical to the look, feel and function. However, a number of these additives are known skin allergens. Will these chemicals leach out when the device is being used as intended? How does an innovator answer these questions and determine if their products are safe?
In this study, we illustrate a path to answer some of these concerns. Wristbands and safety goggles were used as representative consumer wearables. These wearables were leached with simulated sweat to simulate what the consumer may be exposed to during device use.
The leachates were analyzed for chemical composition by a host of analytical techniques. Chemical compounds in the leachates were identified using a combination of scientific experience and commercially available spectral libraries. Once the chemical components leached were identified, a team of toxicologists evaluated the chemical test results to determine whether potential risks to consumers were within acceptable limits. These results are indicative of the kind of experiments that are needed to help understand the chemical profiles of wearables and the toxicological risk to the users of these products.
INTRODUCTION
An important concern for any product worn against the skin surface is the potential risk of skin sensitization. Skin sensitization occurs when a chemical with a reactive structure, a skin sensitizer, penetrates the top layers of the skin, and interacts with the immune system in a way that causes production of specialized T cells. The next time the body encounters the chemical, these T cells are primed to react, resulting in an inflammatory response including skin redness, swelling and blistering. The degree of exposure is an important consideration; at sufficiently low levels of exposure, the risk is minimal. The specific safety threshold will be specific to the chemical and particular exposure conditions. If exposures exceed safe levels, the resulting chemical sensitivity can persist for decades and can leave the individual sensitive to any product that contains the sensitizing chemical. Due to its chronic nature, potential skin sensitization is a growing health concern among dermatologists and consumer product regulators.
Consumer wearables lack regulatory guidance on how to measure safety. So how do we design studies to ensure consumer safety? We can use information from regulated product safety testing to guide us through the non-regulated waters. Testing protocols for medical devices, which are a regulated product, can be used for guidance. Wearables, when compared to medical devices, are similar in that the materials of construction are in direct contact with the consumer and thus pose a similar threat in regard to safety. The medical device industry has very stringent guidelines that need to be met prior to the device coming to market. These guidelines ensure that materials of toxicological concern are not coming into contact with the body during use of the medical devices. Extractable and leachable studies are common experiments used to evaluate a medical device for consumer safety.
Consumer wearables may contain various additives such as plasticizers, UV stabilizers, pigments or antimicrobial agents in addition to the base polymer material, many of which are skin sensitizers. The choices made in materials of construction leads to concerns about chemical exposure. Extractable/leachable studies are necessary to help understand the chemical profiles of many wearable products and the toxicological risk to users of these products. This can be a proactive measure to avoid harm to users of the wearable products and avoid product recalls, legal suits, and the attention of consumer product regulatory agencies.
The following samples were evaluated as representative consumer wearables.
- Black Wristbands, Adult
- Pink Wristbands, Adult
- Anti-Fog/Anti-Scratch Safety Goggles
The wristbands (i.e., silicone-based fashion bracelets) that were analyzed are pictured in Figure 1.

The safety goggles, pictured in Figure 2, are composed of three main components: the band, the mask, and the lens, all constructed from a different polymer. These components were leached in a simulated body sweat solution. The simulated sweat leachate was then analyzed by chromatographic methods such as Gas Chromatography and spectroscopic methods such as Inductively Coupled Plasma Mass Spectrometry to identify organic and inorganic components that may leach out into sweat. The data from this analysis was then reviewed by toxicologists to determine if any of the chemical components may cause skin sensitization.
RESULTS
Chemical assignments were made for the peaks detected using scientific knowledge in conjunction chemical databases. Examples of compounds identified include:
- 3-buten-1-ol, 3-methyl-
- formamide, N,N-dimethyl-
- 4-hexen-3-one, 2,2-dimethyl-
- 2,5-hexanedione
- 2-pyrrolidinone, 1-methyl-
- 2,5-hexanediol, 2,5-dimethyl-
- 1,3-dioxan-5-ol, 4,4,5-trimethyl-
- 1-butanol, 3,3-dimethyl-
- 1,8-nonanediol, 8-methyl-
- p-dioxane-2,5-dimethanol
- triacetin
- butylated hydroxytoluene
- dodecanoic acid, 1-methylethyl ester
- benzophenone
- Tridecylbenzene sulfonic acid
- dodecanoic acid
- dioctyl Sodium Sulfosuccinate
- dodecanamide, N,N-bis(2-hydroxyethyl)-
- tetradecanoic Acid
- gamma-linolenic acid
- pentadecyl acrylate
- IPPD (N-isopropyl-N’-phenyl-p-phenylenediamine)
- octamethylenediamine and 3,3′-dimethoxy-4,4′-biphenylene diisocyanate oligomer
- 4-tert-butyl-2-(hydroxymethyl)phenol oligomer
- bisphenol-A carbonate oligomer (dihydroxy terminated)
- polyethylene glycol oligomer
- polyglycerin and polydodecanedioic acid oligomer
TOXICOLOGICAL REVIEW AND RISK ASSESSMENT
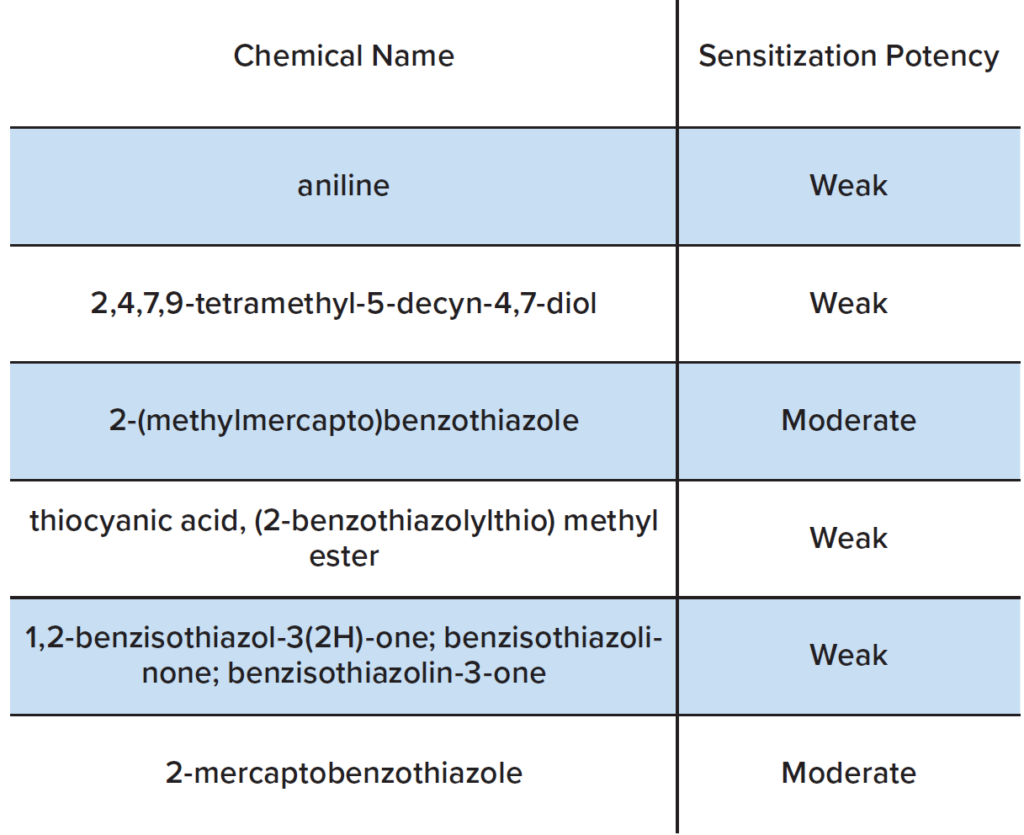
Once the chemical analysis data was in hand, a review was conducted to assess the potential of these chemicals to produce skin sensitization. If the chemicals were identified as potential skin sensitizers, calculations were made to determine the amount that would correspond to an acceptable risk. We consulted various publicly available toxicological databases, including European Chemicals Agency (ECHA) dossiers, the US National Toxicology Program’s Local Lymph Node Assay (LLNA) database, US EPA chemical registration documents, and specific skin patch testing references. In cases where no specific data were available for a particular chemical, structurally similar chemicals were used to establish skin sensitizer status and potency (i.e., read-across). In cases where no reasonable analog chemicals with reliable sensitization hazard data were identified, we relied on expert rule based in silico programs to estimate skin sensitization hazard. Most of the chemicals detected in the extracts from the wrist bands or goggles were not found to be skin sensitizers. There were no sensitizers identified in the wristbands. Notable sensitizers identified in the goggles are summarized in Table 1.
CONCLUSIONS
The extractable/leachable studies described above evaluated potential wearable safety issues for the consumer. Wristbands and goggles were used as examples of consumer wearables. Leaching experiments were performed on these items using simulated sweat. The chemicals present in the leachates were identified using Gas Chromatography and Inductively Couple Plasma Mass Spectrometry. A toxicological review of the chemicals was performed, several notable weak and moderate sensitizers were detected in the leachate from the goggles.
With these findings, additional testing is necessary to quantify the amount of sensitizers that was released. The toxicological team will, in parallel, determine an exposure limit for each of the sensitizers. The amount of sensitizer in the leachate is then compared to the exposure limit provided by the toxicologists. If the quantity of a sensitizer is well below this limit, the wearable is considered “safe.” Conversely, if the quantity of a sensitizer is well above this limit, the wearable is “of toxicological concern.”
There are many steps in the lifecycle of a product including prototypes, raw materials procurement, finished product models, and retail manufacturing. The study presented here can be used to evaluate potential safety issues along every step of the lifecycle. This can be a proactive measure to avoid harm to users of the wearable products and avoid product recalls, legal suits, and the attention of consumer product regulatory agencies.
REFERENCES
Api, A.M.; Basketter, D.A.; Cadby, P.A.; Cano, M.F.; Ellis, G; Gerberick, G.F.; Griem, P.; McNamee, P.M.; Ryan, C.A.; Safford, B., “Dermal Sensitization Quantitative Risk Assessment (QRA) for Fragrance Ingredients.” Regul. Toxicol. Pharmacol. 2008 52 (1), 3-23.
Would you like to learn more about Extractable and leachables?
Contact us today for your Extractable and leachable needs. Please complete the form below to have an EAG expert contact you.