Why Do Toothbrush Bristles Harden Over Time?
Home » Why Do Toothbrush Bristles Harden Over Time?
With prolonged use, toothbrush bristles may begin to feel hard, stiff and rigid when compared to an unused toothbrush.
To investigate why, toothbrushes under different use conditions were analyzed using a variety of analytical techniques to compare the bristles’ chemical composition, examine their visual appearance, and characterize residual deposits.
SAMPLES
Two different toothbrush use conditions were selected: (1) a toothbrush used twice a day for 7 consecutive days (approximately 14 uses) and (2) a toothbrush used intermittently over the course of six months (approximately 24 uses). A new, unused toothbrush was included in the comparison as a control.
The toothpaste was also analyzed as a reference material, since there was a high probability that residual material may remain on the brush after use. The samples were designated as follows.
| Sample # | Sample Description |
| S1 | New unused toothbrush (control) |
| S2 | Toothbrush used weekly for 6 months (approximately 24 uses) |
| S3 | Toothpaste (same brand used during toothbrushing) |
| S4 | Toothbrush used for 7 days (approximately 14 uses) |
FOURIER TRANSFORM INFRARED SPECTROSCOPY (FTIR)
FTIR was used to determine the chemical family of bristles from each corresponding toothbrush using an attenuated total reflection (ATR) sampling technique. ATR has the advantage of simplicity in enabling samples to be analyzed in their natural state with minimal sample preparation. FTIR spectroscopy is able to obtain information on the types of chemical functional groups that make-up a material based on the frequency at which bonded atoms vibrate when absorbing infrared light. FTIR can both confirm the identity of the synthetic polymer that makes up the toothbrush bristles as well as detect percent-level contaminants, such as toothpaste residue. The spectral fingerprint of the toothpaste was also collected as a reference material, to aid in interpretation of the data.
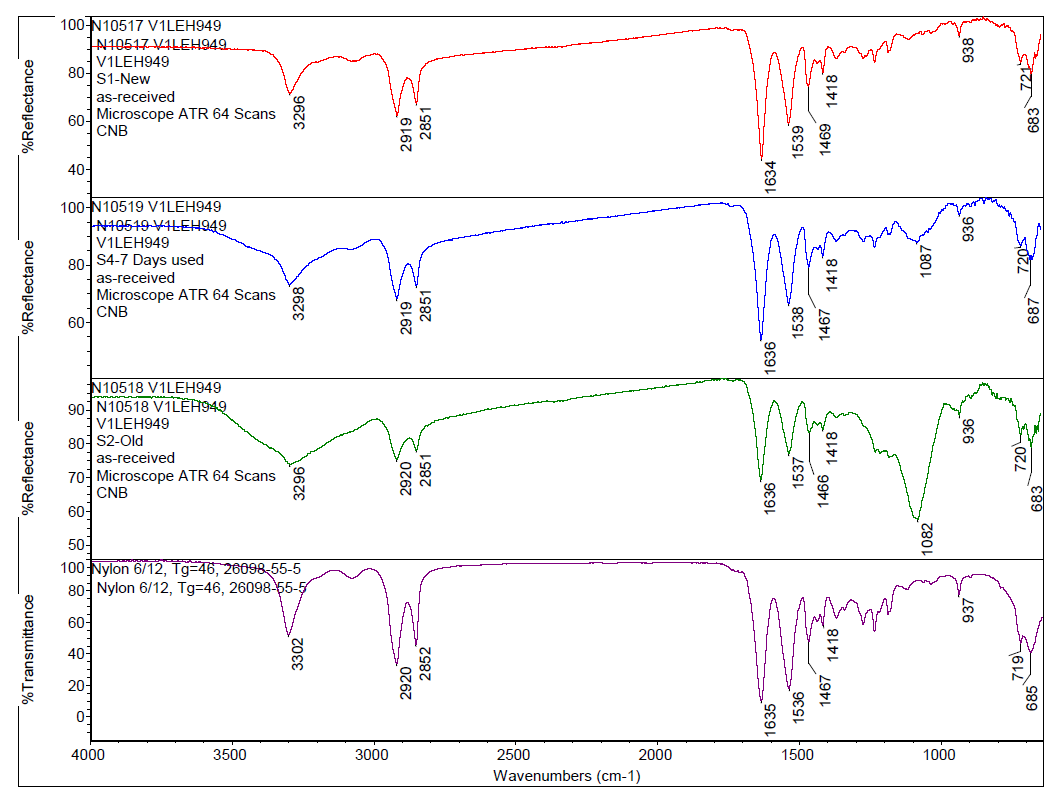
By applying a computer-assisted search algorithm of the commercial library, it was determined that the toothbrush bristles were composed of Nylon 6/12 material, as can be seen in Figure 1. Comparing the bristles from different toothbrushes to one another, the spectra look similar by FTIR, but the peak centered at 3296 cm-1 broadens for toothbrush specimens used for longer periods of time. Additionally, a peak around 1082 cm-1 appears in the used bristles and increases in intensity after extended use, as can be seen in Figure 2. Comparing these spectral features observed in the used toothbrushes to the toothpaste reference spectrum confirms the used toothbrushes contain toothpaste residue. This indicates that even after rinsing with tap water, some toothpaste remains within the toothbrush bristles and the amount of toothpaste in the bristles increases with prolonged use.
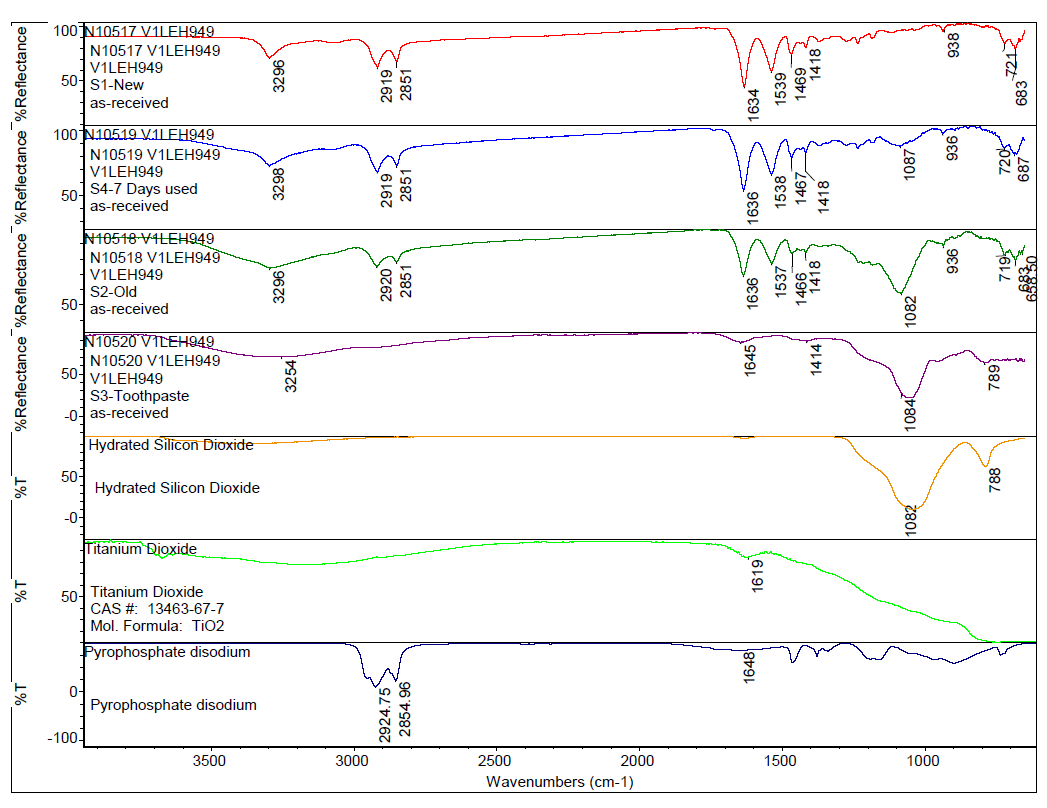
To gain additional insight into what specific information can be concluded from the FTIR data, a list of ingredients from the toothpaste was obtained and corresponding reference spectra were selected from the commercial library for comparison. Among the ingredients listed, FTIR spectra of selected ingredients such as disodium pyrophosphate, hydrated silica, and titanium dioxide were cross-referenced to determine which toothpaste ingredient was responsible for the intense peak at ca. 1082 cm-1. The stacked plot of spectra in Figure 2 suggests that a major ingredient that is being retained on the bristles after use is from hydrated silica, which is formulated into toothpaste to function as a fine gel abrasive to aide in plaque removal.
SCANNING ELECTRON MICROSCOPY (SEM)
Scanning electron microscopy (SEM) can be used as a tool to visualize and compare microscopic structures within various materials. In SEM, a focused electron beam scans over the surface of the sample, and a response is generated based on electrons’ interactions with individual atoms that compose the material. Depending on the type of electron interaction being monitored, the corresponding images convey information about the topography and composition of the sample. For this application, both the toothbrush bristles (Nylon) and the toothpaste residue are non-conductive materials; thus, in order to obtain a well-resolved image, the bristles were sputter coated with a thin layer of metal (gold) to render the surfaces electrically conductive.
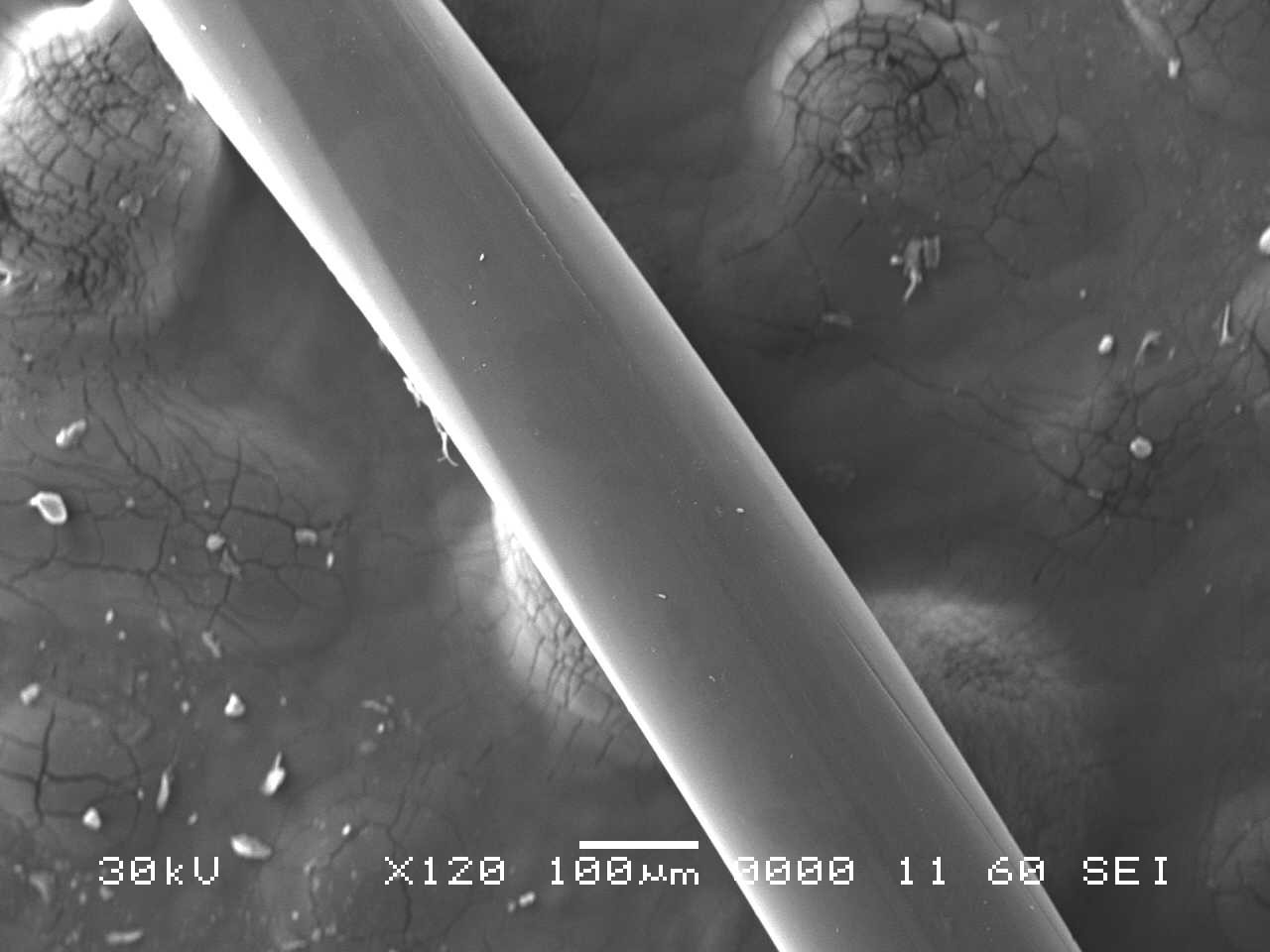
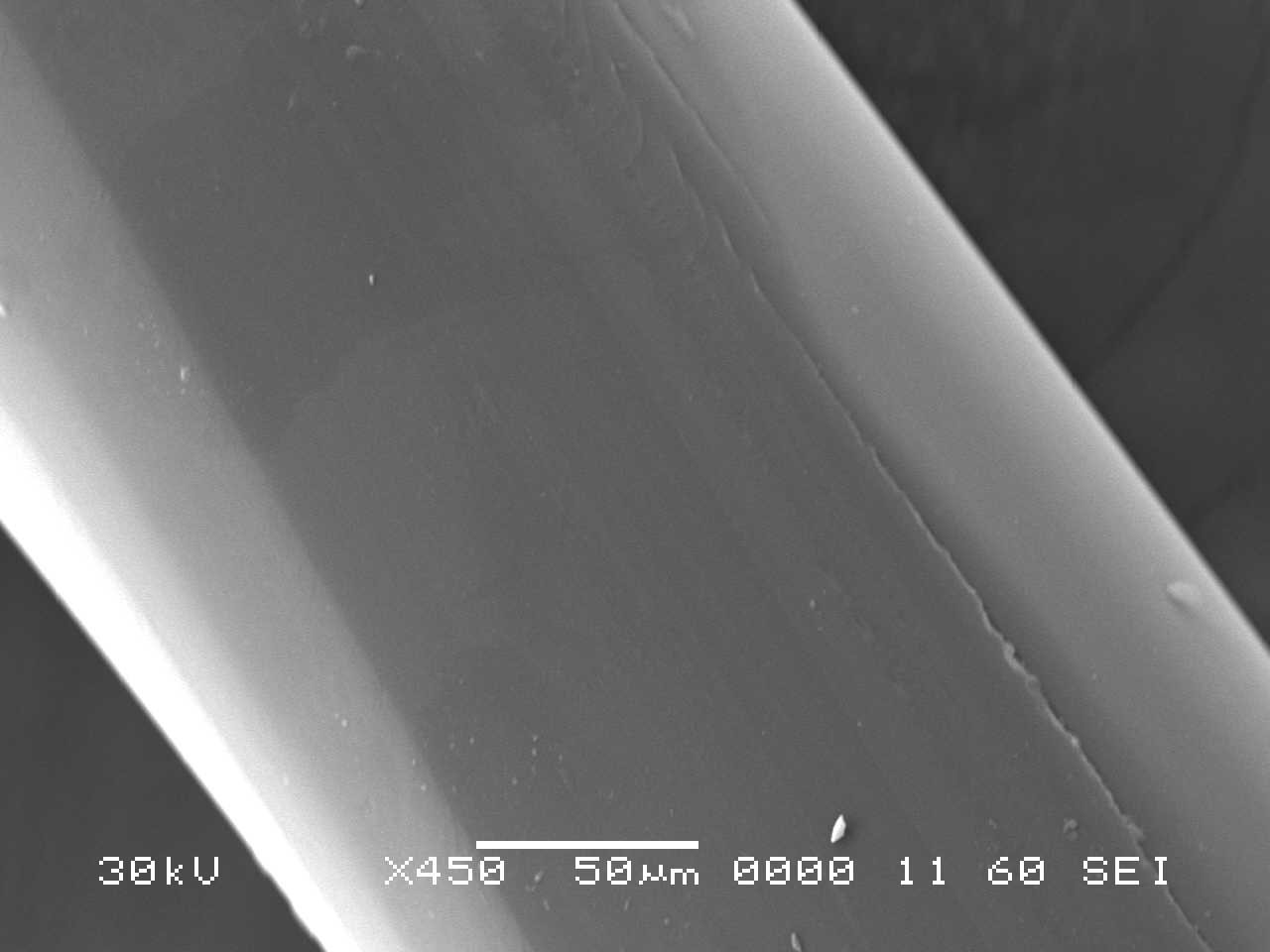
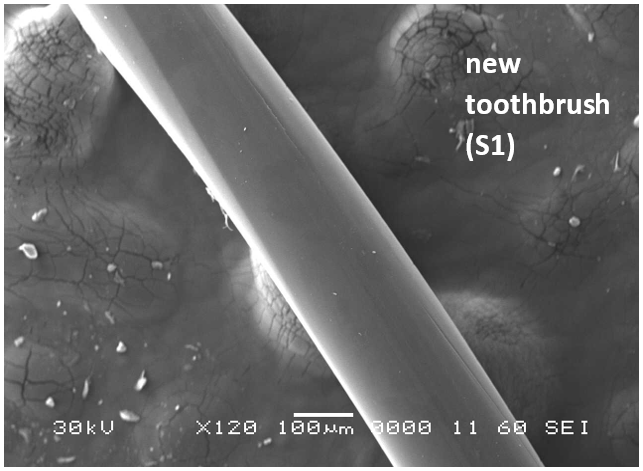
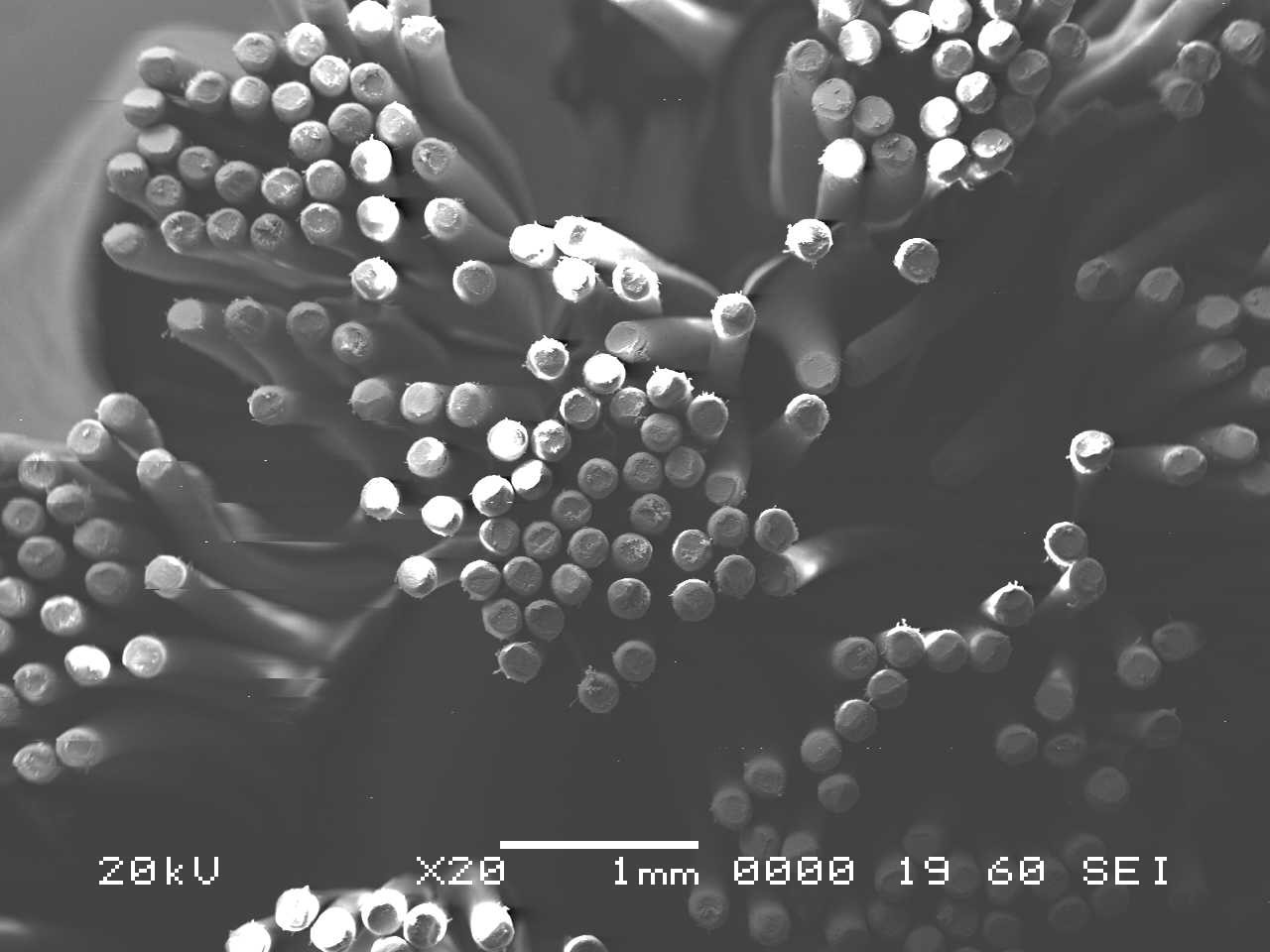
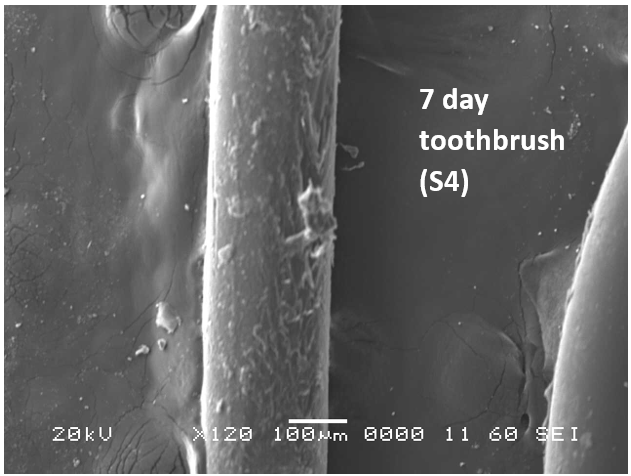
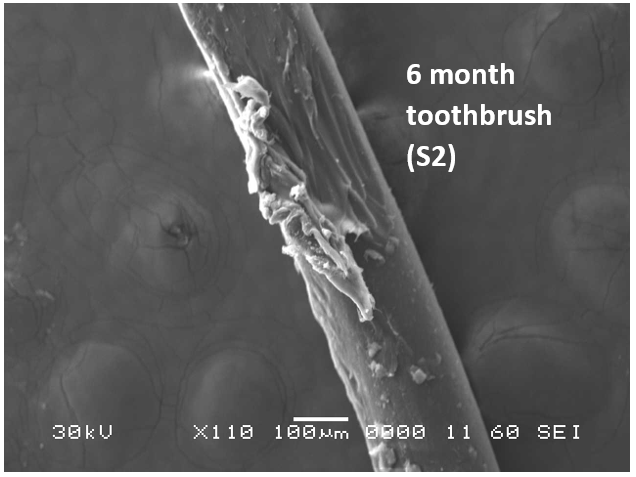
Analysis by SEM reveals that, as expected, the new control toothbrush bristle (S1) is smooth and does not have apparent particulate matter (Figure 3). A lower magnification SEM image of the full toothbrush head shows bristles that are separate and able to freely move. The 7-day intermediate use toothbrush (S4) begins to show wear on the surface of the bristles, and in a lower magnification image, some individual bristles are starting to adhere to one another (Figure 4). After six months of use, the oldest toothbrush bristles (S2) display extensive fraying and particulate matter (Figure 5). Images of the full toothbrush head exhibit some groups of bristles stuck or “glued” together and packed in columns. A comparison of all three toothbrushes is provided in Figure 6.
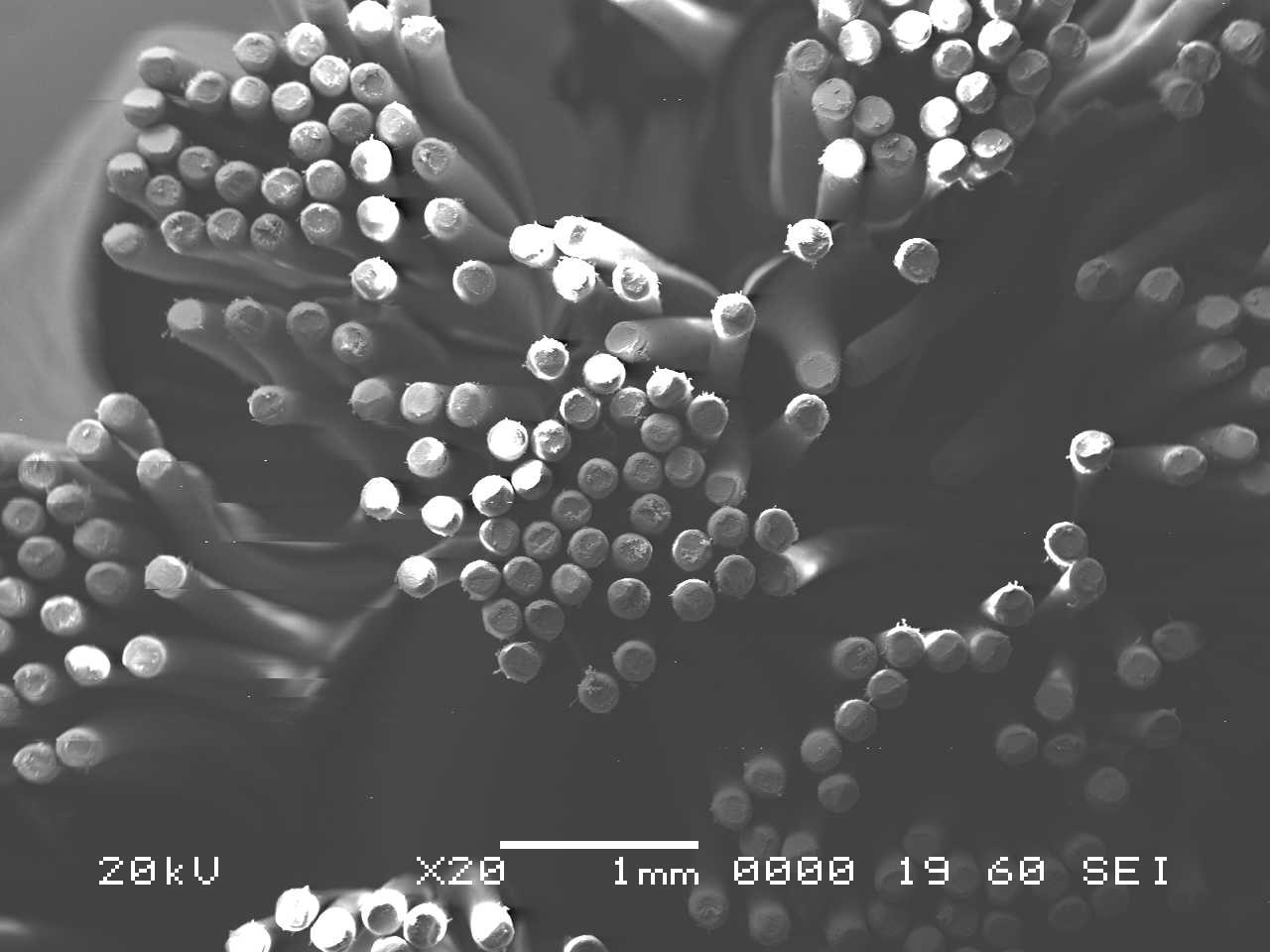
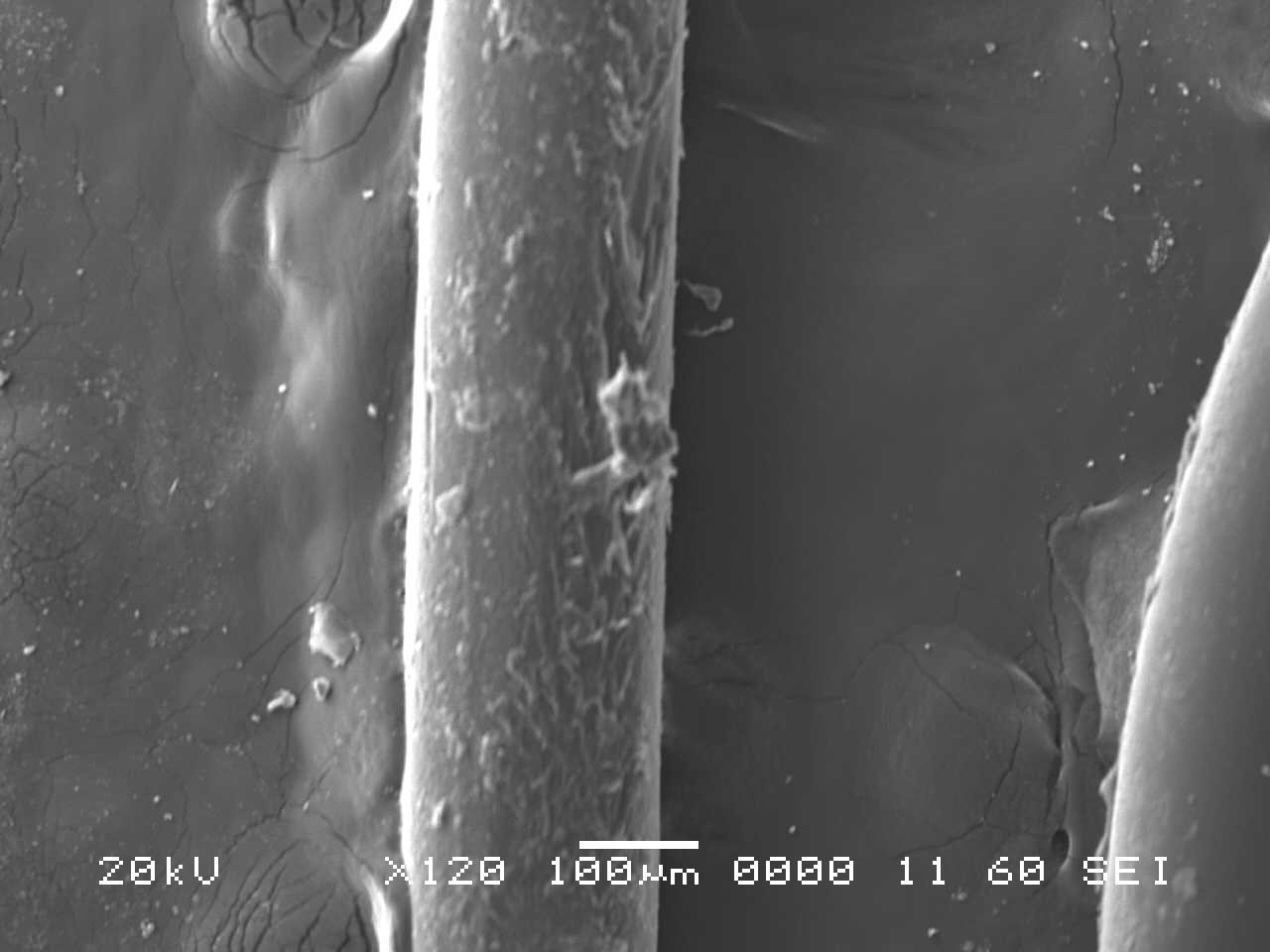


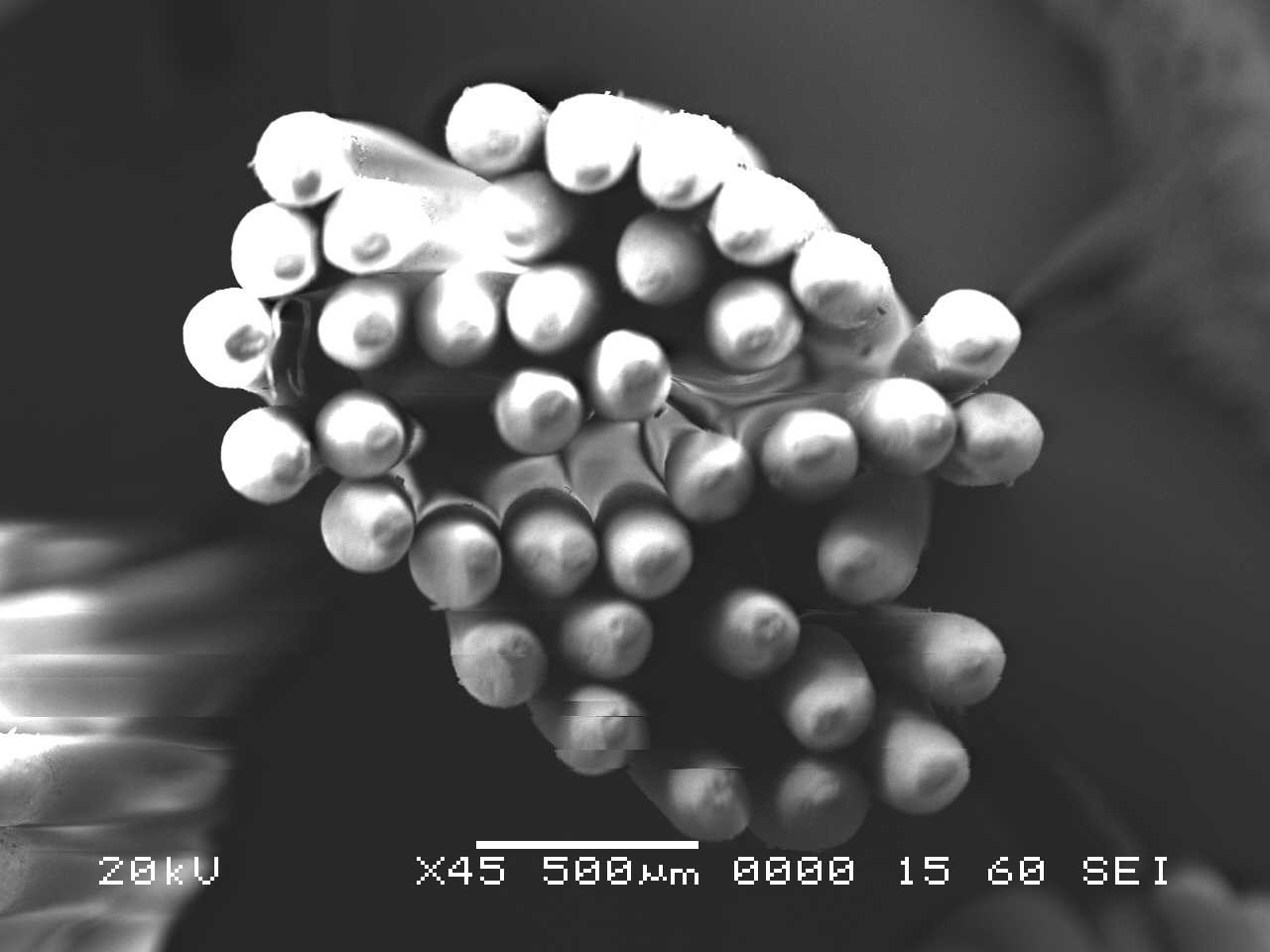



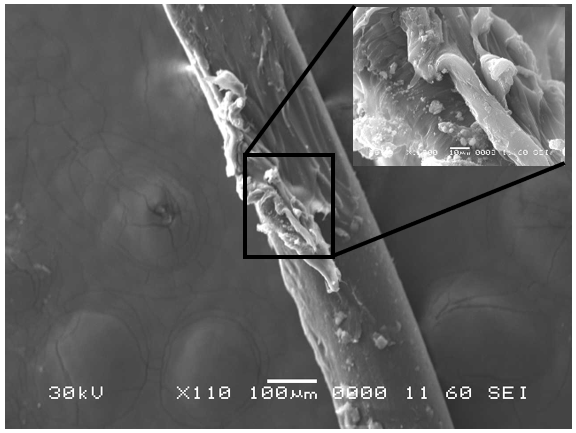








THERMAL GRAVIMETRIC ANALYSIS (TGA)
In thermal gravimetric analysis (TGA), a sample is heated and the corresponding weight change is measured as the material undergoes thermal events. The amount of inorganic material in the sample can be estimated as the weight percentage of ash that remains after the sample has undergone thermal decomposition.
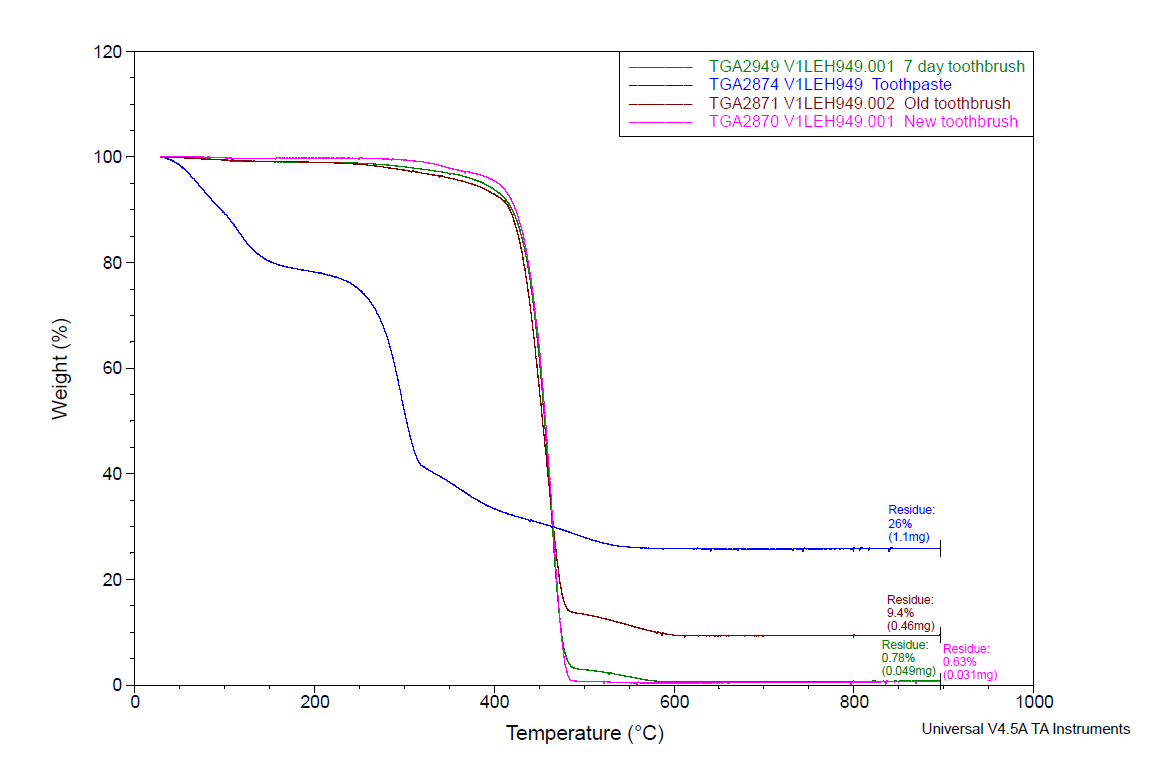
New and used toothbrush bristles were analyzed by TGA from room temperature to 900°C. After heating, the data indicated that the used toothbrush bristles contained a much higher amount of residual inorganic material. This data is consistent with the FTIR spectra and SEM images, which indicated the presence of inorganic material in the form of toothpaste, is present in and around the bristles. The difference in the TGA curves of the new toothbrush (S1), 7 day old toothbrush (S4), six month old toothbrush (S2) and toothpaste sample can be seen in Figure 7. There is much greater residue in the six month old toothbrush (S2=9.45% residue, maroon trace) than in the toothbrush after 7 days of use (S4=0.78% residue, green trace) and the new toothbrush (S1=0.63% residue, pink trace). Since TGA only provides gravimetric and not compositional information on the residue, another analytical technique – energy dispersive X-ray spectroscopy (EDS) – was performed on the TGA ash to determine its elemental profile.
ENERGY DISPERSIVE X-RAY SPECTROSCOPY (EDS) OF TGA ASH
To gain insight into the chemical composition of the residue that remained following the TGA experiment, an elemental profile was collected on the ash using an energy-dispersive X-ray detector inside the scanning electron microscope. As the high-energy electron beam scans over the sample, this initiates a series of steps within the atom that ultimately result in an x-ray being emitted. The energy of this x-ray is characteristic of the specific element that it originated from. By detecting and measuring the X-rays that are emitted, we are able to determine the elemental profile of the ash.
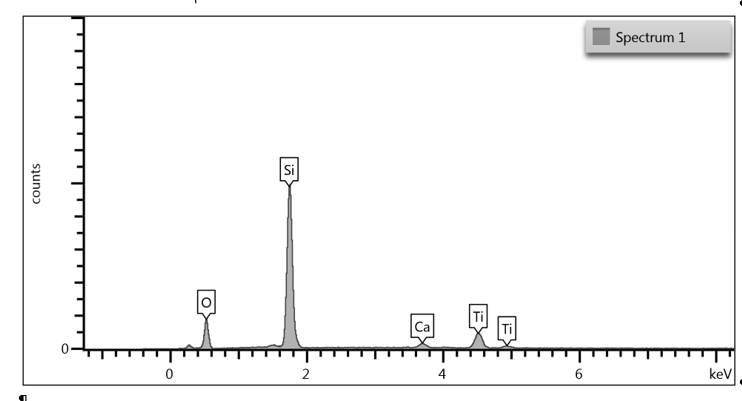
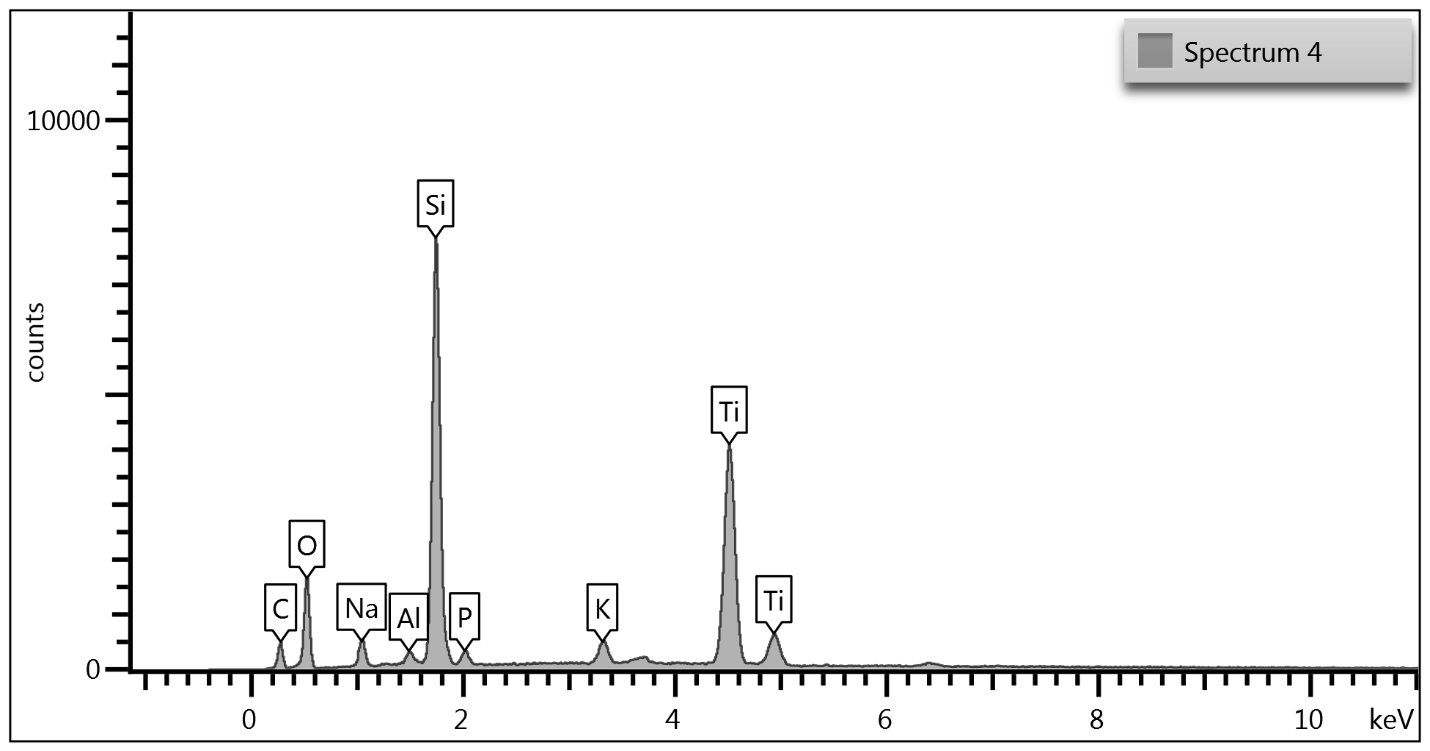
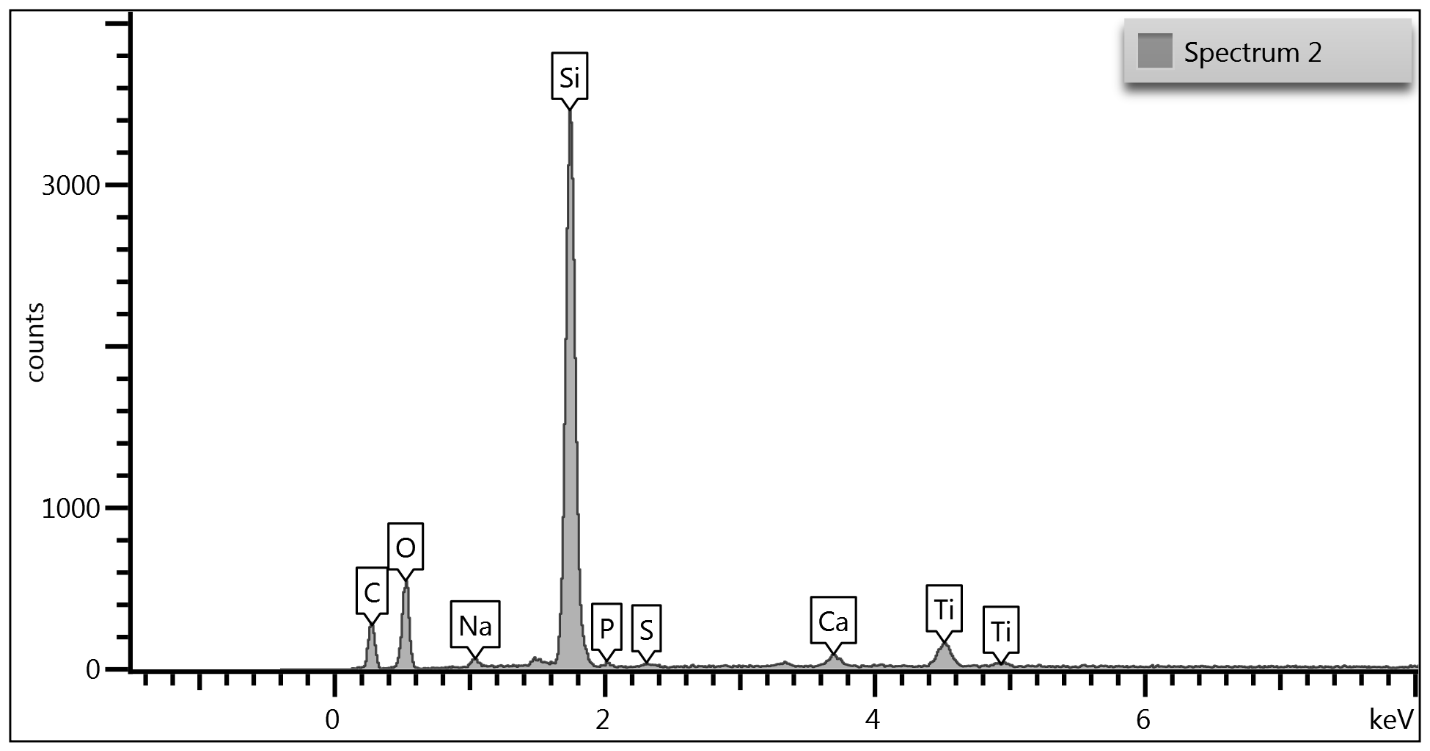
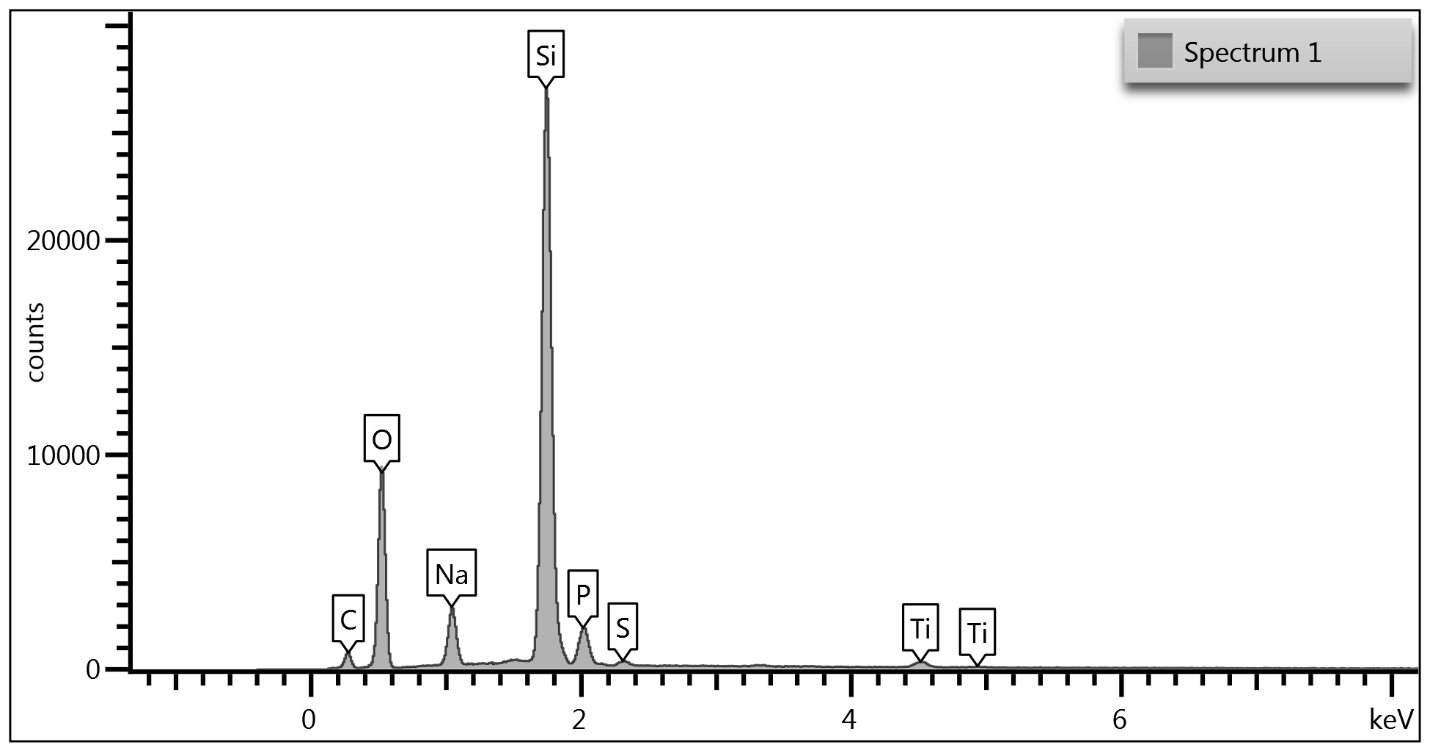
Upon examination of the data, it is determined that EDS of the TGA ash samples (Figure 8 – Figure 10) and the toothpaste samples (Figure 11) contain mostly the same elements. The EDS of the ash from bristles of S1 (Figure 8) suggests that silicon (Si) and titanium (Ti) are also present in the new control toothbrush, likely due to pigments formulated into the Nylon bristles. This complicates the analysis, as coincidentally there is also hydrated silica and titanium dioxide that are ingredients in the toothpaste itself that would be useful as “marker compounds” for the presence of toothpaste.
However, It is important to note from the TGA data, that the ash remaining from the new toothbrush itself is minimal (0.63%) but is significant (26%) in the toothpaste. Thus, <1% of residue would be attributed to the bristle material itself; since the TGA residue of the six-month use toothbrush (S2) is over 9%, and the most intense peak is silicon (Si), it makes sense, in conjunction with other data, that extra silicon-containing material is due to hydrated silica from toothpaste residue on the bristles. The presence of sodium (Na), phosphorus (P), and sulfur (S) are also consistent with other ingredients from toothpaste. The toothbrush used for 7 days (S4) only had 0.78% ash; this just slightly exceeds the new control toothbrush (S1). Thus, the TGA and EDS data suggest that the intermediate use toothbrush (S4) bristles should not be as hard, since there is not as much residual toothpaste coating the bristles.
CONCLUSION
A multi-technique approach was employed to investigate a common phenomenon that many of us experience – why toothbrush bristles get hard over time. FTIR and EDS indicate toothpaste residue accumulates as the duration of use increases. Electron microscopy images show the bristles becoming mechanically damaged and increasingly “glued” together by residual toothpaste over longer use periods. Data from TGA shows that there is significantly more toothpaste residue that builds up on bristles over the course of 6 months of use vs. a toothbrush that has only been used for 7 days.
Several conditions contribute to the bristles becoming hard over time. The mechanical action from brushing, in the presence of a toothpaste coating, causes the Nylon bristles to begin to fray and break down. Over time, this can cause the bristles to become adhered to one another and renders the act of rinsing the toothbrush with tap water at the conclusion of brushing less effective. These factors ultimately result in the bristles becoming stiff and hard and less effective for the user, and usually signals it is time to acquire a new toothbrush!
Would you like to learn more about Why Do Toothbrush Bristles Harden Over Time?
Contact us today for your analysis of toothbrush bristles. Please complete the form below to have an EAG expert contact you.