Corrosion Resistance Study of Electropolished Medical Devices
Home » Corrosion Resistance Study of Electropolished Medical Devices
Medical devices such as stents, catheters, and heart valves need to be resistant to corrosion to avoid adverse effects after deployment in the human body. Optimization of the medical device corrosion resistance is therefore critical. Furthermore, supporting data that a medical device is sufficiently resistant to corrosion is required by the FDA for approval of the device.
Auger Electron Spectroscopy enables both measurement of the surface oxide layer thickness and investigation of the oxide layer uniformity of electropolished medical devices. Auger can be used to provide representative data from the entire device or localized data to highlight differences between various areas on the surface(s) of interest. Figure 1 shows a typical Auger Depth Profile.
There are three major applications to this:
- Controlling the uniformity of the electropolishing process (process control)
- Investigating treatment effects and environmental exposure (including deployment in model environments)
- Investigating discontinuities or design features such as wear marks, welds or defects
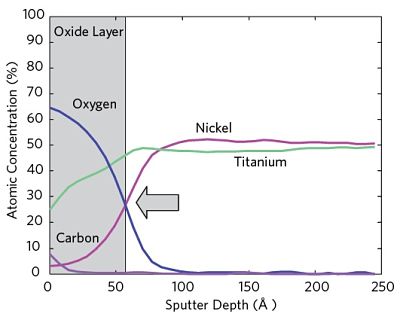
Figure 2 shows the oxide layer thicknesses, as measured from different components of several medical device samples after electropolishing. While some minor differences are observed between the components, the overall uniformity of the electropolishing process is satisfactory.
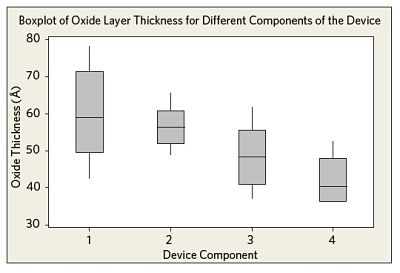
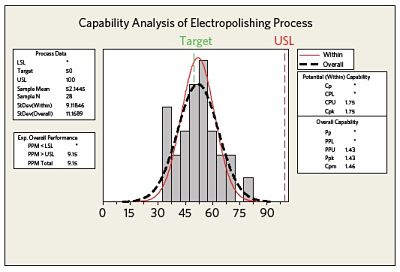
Figure 3 summarizes the observed process capability and compares the within subgroup variation (data from within the different areas of the device) to the overall process variation. In this case only an upper specification limit has been specified. Both the Cpk and Ppk values indicate good process capability/performance.
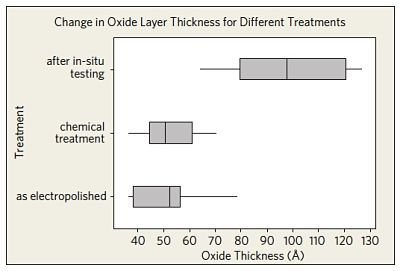
The effect of different treatments on the oxide layer thickness is shown in Figure 4. The observed passivation layer thickness after in-situ tests of a group of devices in an accelerated human model environment led to a statistically significant increase in the oxide layer thickness (compared to the as-electropolished values and the values measured after chemical treatment).
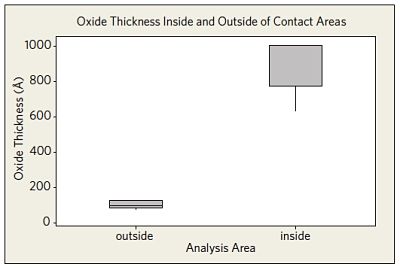
Auger Electron Spectroscopy was also used to analyze the oxide layer thickness in specific metal to metal contact areas of the in-situ tested devices. The thicker oxide in areas of metal to metal contact showed the human model environment may lead to localized surface modifications (Figure 5).
Would you like to learn more about Corrosion Resistance of Medical Devices?
Contact us today for your corrosion resistance of electropolished medical device needs. Please complete the form below to have an EAG expert contact you.