
Cryo-FIB/SEM: Enhancing Precision and Minimizing Damage in Material Analysis
Cryogenic focused ion beam and scanning electron microscopy is a technique used to reduce damage caused by ion and electron beams.
Home » The Possibilities of Graphite: From Pencil “Lead” to Lithium-ion Batteries
Graphite is and has been an ubiquitous mineral in our lives, from pencil “lead” and water filters to ancient markings on pottery. Natural graphite mining is a rapidly expanding source of high-quality graphite for industrial use. Synthetic graphite is the other source of graphite; however, the high cost of production has refocused efforts on mining of natural graphite. Graphite is most often associated with metamorphism of carbon-containing sedimentary rocks, or in hydrothermal veins. it is sourced from China, India, Brazil, and Canada, among other places. Graphite is widely used as an anode material in lithium-ion batteries, aerospace, nuclear, refractory materials, steel production, lubricants, and many other applications.
Graphite is categorized into 3 groups: lump, flake, and amorphous. Lump graphite is the highest grade and rarest natural graphite, with amorphous graphite being the lowest grate and most abundant. Flake graphite currently constitutes roughly 49% of all mined graphite consumption due to its good crystallinity and purity (80-95%

carbon content). There is an essential need for the lithium ions to be able to freely and efficiently travel into and out of the anode to ensure safe, and long-lasting operation of the batteries. For this the carbon chemical purity requirement is better than 99%.
There are many ways to refine and purify graphite, but two general categories are hydrometallurgical, and pyrometallurgical purification. The cheapest method to purify graphite is the process of floatation, by which the graphite goes through a series of grinding and froth floatation in order to physically separate the hydrophobic graphite from the hydrophilic gangue. Other methods that fall into the hydrometallurgical category include acid-base and hydrofluoric-acid purification methods, which leverage carbon’s chemical inertness to chemically purify graphite. Pyrometallurgy includes chlorination roasting and other processing methods, during which the graphite is heated up to high temperatures under a variety of conditions for removing impurities.
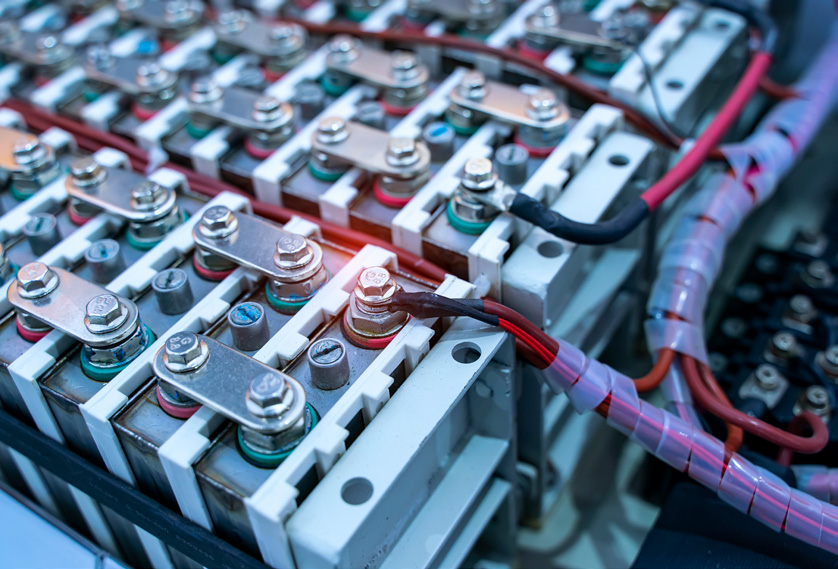
At Eurofins EAG Laboratories (EAG) we offer a wide range of analytical testing services to provide the purity testing of graphite for the most demanding applications – like electric vehicle batteries. The Glow Discharge Mass Spectrometry (GDMS) technique is very robust, stable, and can provide analysis rapidly. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) is a fully quantitative technique for acquiring matrix-level (1000 ppm to weight-percent) constituents and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) is a fully quantitative technique that is able to detect trace-level analytes. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS) is a micro analytical solid-state technique. It is useful in being able to analyze specific
locations or map the surface of a material; with matrix-matched standards, it can provide fully quantitative data. Lastly, Laser Induced Breakdown Spectroscopy (LIBS) is a solid-state technique similar to ICP-OES and looks at the emissions spectra of elements.
Whatever your testing needs are, EAG offers a suite of complementary techniques serving industries like aerospace, defense, and semiconductor manufacturing. EAG is a one-stop-shop for purity analysis of graphite anode materials used in advanced battery production. Contact us today to learn more.

Cryogenic focused ion beam and scanning electron microscopy is a technique used to reduce damage caused by ion and electron beams.

Aerospace and defense government suppliers can have confidence in EAG’s credibility in providing accurate and high-quality testing services.
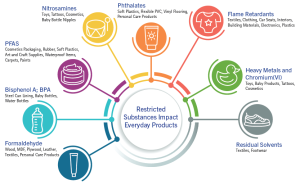
As the list of restricted substances grows, testing demands customized methods to identify issues early. This is a complex issue that requires a strategic approach.

FA plays a critical role in product development and improvement. During this live Ask the Expert event, we will answer pre-submitted questions from our audience about electronic device failure analysis.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.