
Cracking of Molded Components
An injection molded component in a consumer product was found to have an increased failure rate over a three-month period.
Home » The Conspicuous Case of the Missing T0 in ISO 10993-5 Cytotoxicity Testing
In the world of medical device safety testing, ISO 10993-5 plays a crucial role. This standard outlines the procedures for evaluating the cytotoxicity of medical device materials, ensuring they are biocompatible and safe for use in human applications. A key aspect of this, which is sometimes overlooked in certain studies, is the T0 time point, or time zero. Missing T0 can create significant problems in the interpretation of cytotoxicity data.
ISO 10993-5 is a part of the larger ISO 10993 series, which is a set of standards guiding the biological evaluation of medical devices. Specifically, ISO 10993-5 focuses on in vitro tests for cytotoxicity, assessing whether extracts from medical devices cause damage to or kill cells.
Cytotoxicity testing is generally performed by exposing cultured mammalian cells to test materials or extracts. The cells are then observed over a specific period to assess the effects, such as cell death, changes in morphology, or inhibition of cell growth.
In any time-dependent biological assay, the T0 time point—also known as baseline or initial condition—is crucial. It represents the condition of the cells or the system at the exact moment the test material is introduced. Without T0, it’s impossible to establish a proper reference for subsequent observations. In ISO 10993-5 cytotoxicity testing, T0 provides the baseline for comparing how cells change over time in response to the material under investigation.
However, in practice, many studies either fail to report the T0 time point or entirely omit it. This omission can create gaps in the reliability of the results.
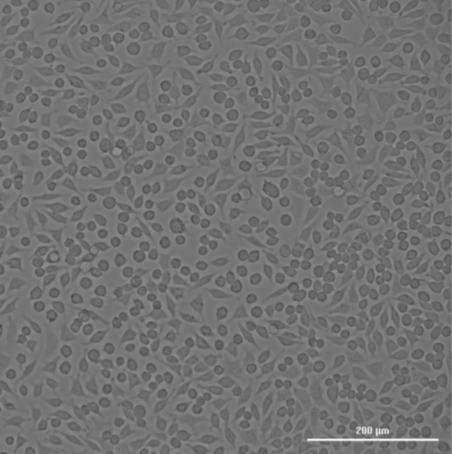
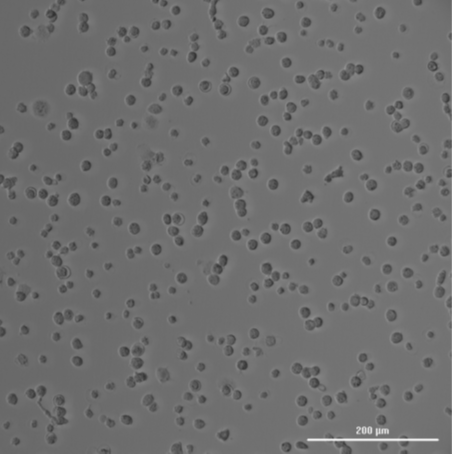
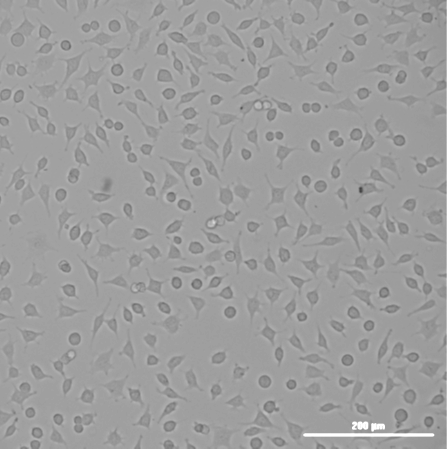
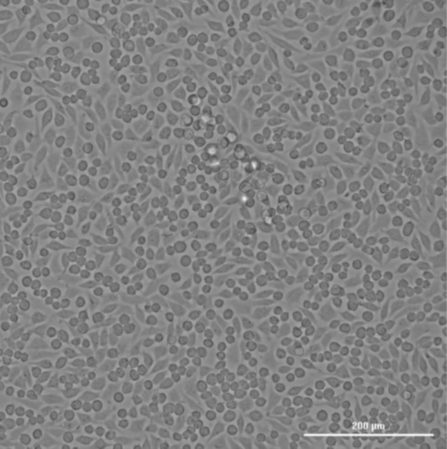
The missing T0 in ISO 10993-5 testing can lead to misleading interpretations of the cytotoxicity of a material. Here are some key reasons why T0 is critical:
ISO 10993-5 and other regulatory standards emphasize the importance of reproducible and reliable data. Without T0, test results may fail to meet these stringent regulatory requirements, jeopardizing the device’s path to approval. Additionally, reproducibility across different laboratories becomes problematic if critical initial conditions are missing.
Consider a scenario where a medical device is being tested for cytotoxicity. The cells are exposed to an extract of the device, and after 24 and 72 hours, their viability is measured. If the T0 measurement is missing, the test might show a significant drop in cell viability at 24 and 72 hours, which could be attributed to the extract being highly toxic.
However, what if the cells were already dying or stressed at T0 due to handling, suboptimal culture conditions, or other unrelated factors? Without a T0 reference, the conclusion that the material is cytotoxic may be entirely inaccurate. In reality, the material might be biocompatible, and the observed effects might be due to a pre-existing issue with the cells.
To avoid the pitfalls of missing T0, laboratories should:
The case of the missing T0 highlights a common but critical oversight in ISO 10993-5 cytotoxicity testing. Without this time-zero baseline, the reliability of your data can be compromised, leading to misinterpretation of the cytotoxicity of medical device materials.
In a regulatory environment that demands precision and reproducibility, including T0 in your cytotoxicity assays is essential. By ensuring that your tests include and report the T0 baseline, Eurofins EAG can provide clearer, more accurate data and avoid potential regulatory setbacks.

An injection molded component in a consumer product was found to have an increased failure rate over a three-month period.
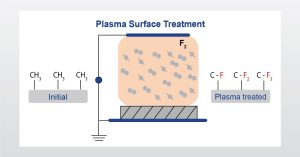
In the full webinar we will discuss how the surface properties of polymers are altered by plasma treatment using characterization techniques

XRD analysis provides identification of crystalline phases present. For 3D printed materials that come from a matrix of multiple elements, it can be unclear whether the cooling creates one, consistent phase of material or if it separates out into multiple phases.

Low-E Coatings on glass are a high efficiency protection of a glass surface for transparency. These coatings help the glass become energy efficient to allow the sunlight to come in or reflect out.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.