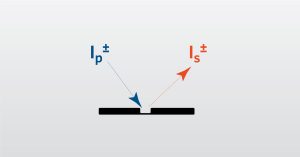
SIMS Webinar
In this webinar we will focus on Dynamic Secondary Ion Mass Spectrometry which used to investigate the elemental structure of materials.
Home » Principles and Strengths of In Vitro Testing
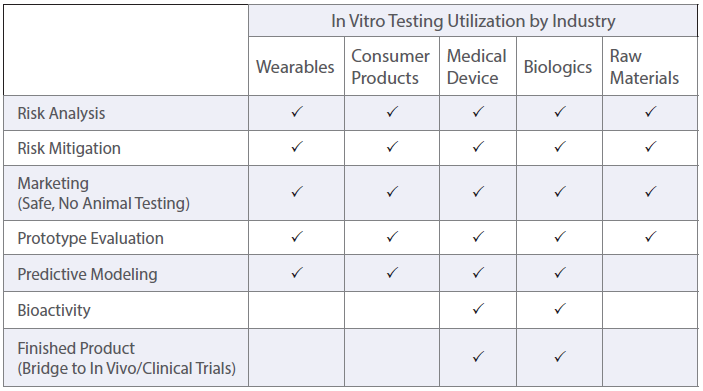
In vitro is the Latin term for “in glass,” meaning that the testing is performed in a container that is outside of a living organism. This testing uses cell-based biological models instead of animals or humans. In vitro efforts help fulfil the FDA’s “3Rs approach” to replace, reduce, and/or refine animal testing.
Replace
Reduce
Refine
In Vitro model systems can be specified to the test sample’s intended use. 2D cell cultures are grown rapidly for high-throughput or multi-sample analysis with minimized variation between treatments. Cell cultures can also be made into 3D tissue models. These models contain differentiated cell layers to physiologically represent complex tissues and evaluate specific endpoints. Using specific geometries, in vitro cell culture models can be used to evaluate simple and complex biological responses.
To assess different biological endpoints, various forms of analytical methods may be implemented. Measurable responses of the in vitro models include cell death, growth inhibition, genetic alterations, changes in surface marker expression and altered metabolism.


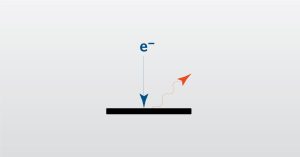
In this webinar we will focus on Dynamic Secondary Ion Mass Spectrometry which used to investigate the elemental structure of materials.
In this webinar we will focus on SEM Cathodoluminescence (SEM-CL) Analyses for Materials such as compound semiconductors
In this webinar we introduce evaluating wearable safety including product recall, lawsuits, and regulatory agency inquiries.

Atomic Layer Deposition technology aids in the demand for computational advancement in the microelectronics industry.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.