
EAG and Eurofins: Cultivating Talent & Community
Join us for an engaging virtual info session titled “Cultivating Talent & Community,” hosted by EAG Laboratories in collaboration with our parent company, Eurofins Scientific.
Home » For Medical Device Testing, Every Variable is Important
The required biological compatibility evaluation of medical devices includes ISO 10993-18:2020 (Part 18) chemical characterization and extractables and leachables testing of medical devices.
For every 10993-18 study performed by a medical device testing lab, a report is generated for a toxicologist to assess, resulting in a toxicological risk assessment (TRA). The toxicologist looks at compounds identified, the confidence level of the identification, and concentration present to perform a risk assessment on the medical device from a chemical viewpoint.
The analytical evaluation threshold (AET), an important part of the extractables and leachables testing in these studies, needs to be calculated for each instrumental technique. The AET is the concentration on an instrument below which analytes do not need to be assessed by a toxicologist per 10993-18.
Proper calculation of the AET is important to successful and timely medical device testing. If the AET is too high, chemical characterization may omit potentially toxic compounds from the chemical characterization report. If the AET is too low, resources are wasted evaluating thousands of compounds at toxicologically irrelevant concentrations.
The goal is to avoid assessing compounds that will be irrelevant in terms of toxicology impact, and make certain that compounds of interest will be evaluated. This is accomplished by ensuring that instrument limits of quantitation (LOQ) are below the AET..
During device submission to regulatory agencies, like the FDA, EU MDR, and other Notified Bodies, reviewers expect the chemical characterization report to demonstrate that the LOQ is below the AET for all techniques.
If the AET calculation is too high, there can be risk to patients, and if the AET is too low, the manufacturer faces higher costs and extended testing time. It can also impact the number of devices needed for a study.
All the variables in the AET equation have equal impact on the final AET number. No variables are far larger or smaller than the others, and none can be ignored or dominate the result.
Some variables can be controlled (number of devices in extraction, extraction volume, dilution factor, uncertainty factor), others cannot (dose-based threshold, patient exposure count). For the best results, the AET must exceed the LOQ for each technique, minimize or eliminate concentration of extracts, and minimize device consumption.
Optimizing experimental device design for the AET:
It is worth restating that every variable is important to making or breaking a Part 18 medical device testing project. The team at EAG Laboratories considers every variable in all methods.
Unlike competitors who provide incomplete data leaving gaps in analysis, EAG provides the deep technical expertise and extensive analytical capabilities to show a fully characterized picture with interpretation of the data. This deeper understanding results in the confidence needed to proceed to a successful regulatory filing, speed up innovation, and reduce failure or litigation risk.
From full chemical characterization including extractables leachables to surface analysis and particulate characterization, EAG supports the Medical Device industry with unmatched capabilities, the most expansive set of instruments in the industry, and fast, flexible problem solving.
Contact us for help with your medical device project.

Join us for an engaging virtual info session titled “Cultivating Talent & Community,” hosted by EAG Laboratories in collaboration with our parent company, Eurofins Scientific.

Our laboratories in France and the Netherlands offer materials testing for industries including semiconductor, consumer electronics, lighting, aerospace and healthcare.

EAG scientists are leaders in the lithium-ion batteries recycling arena. EAG is a valuable resource in understanding these materials.
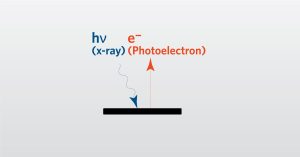
In this webinar we introduce X-Ray Photoelectron Spectroscopy (XPS) which is a surface analysis technique.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.