
ESD Webinar
In this webinar we introduce electrostatic discharge (ESD) testing which is one of the failure mechanisms for integrated circuit parts
Home » Failure Analysis of Medical Device Electronics
Medical device and diagnostics companies have taken great strides in the development of newer technologies to provide better patient assessment and treatments. Devices of the future will require smaller features, lighter materials, and improved stability. Not only is it important to assess the materials and chemistry in medical devices, but it is also equally as important to assess medical device electronics to ensure safety and reliability. From hearing aids to heart pacemakers to catheters and more, advanced electronics can be seen in many of today’s medical devices.
Every day the medical industry lunges forward with more advanced instruments and higher quality consumer products. This new level of sophistication also translates into more complex R&D processes. As the manufacturing process gets more complex, there are many more opportunities for things to go awry. Identifying, diagnosing, and remedying failures becomes dramatically more challenging. Our experienced engineers and scientists possess a comprehensive array of advanced tools and techniques that can perform failure analysis on a large range of electronic devices.
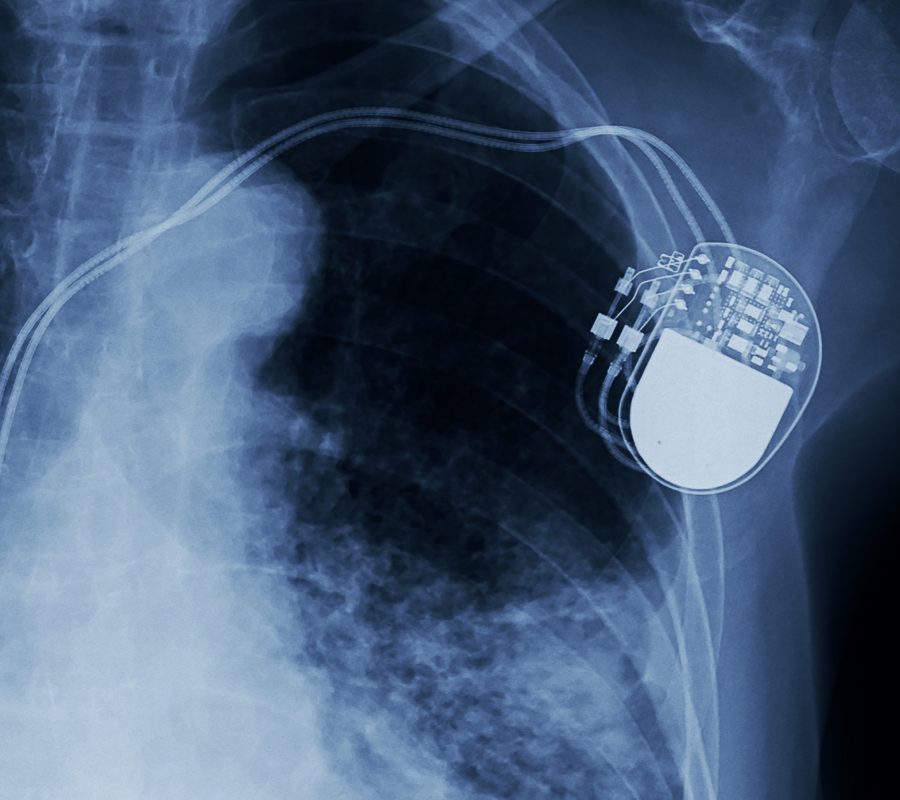

Eurofins EAG Laboratories offers the most diverse and comprehensive suite of testing and engineering support available for medical devices. With failure analysis services that range from non-destructive examination to physical analysis for root cause identification, our failure analysis team has the engineering expertise to help solve problems at any phase of the product cycle. EAG understands the stringent regulations, requirements and expectations of medical devices and are therefore the perfect partners to assist with advancements in the medical device industry.

In this webinar we introduce electrostatic discharge (ESD) testing which is one of the failure mechanisms for integrated circuit parts

During this Ask the Expert webinar, our team of battery materials experts will address your pre-submitted questions during the event, as well as present about our new battery lab’s capabilities.

Nitinol is a material that is widely utilized in the medical device industry for implants due to its unique characteristics; however, this nickel-titanium alloy material also poses the risk of exposure to toxic nickel ions.

Wednesday, March 26, 2025
In this webinar, we will show you how Eurofins EAG supports you in the various phases of reliability testing to come to a robust product, including the different types of actual testing.
To enable certain features and improve your experience with us, this site stores cookies on your computer. Please click Continue to provide your authorization and permanently remove this message.
To find out more, please see our privacy policy.