Structural and Chemical Characterization of Li-ion Batteries
Home » Structural and Chemical Characterization of Li-ion Batteries
Lithium ion batteries have improved rapidly in the last 10 years to become the main power source in portable electronics, telecommunications, and large capacity applications such as in electric vehicles (EV). Ongoing improvements in characterization techniques must also meet industry, regulatory and consumer needs as battery manufacturers and end-users demand higher battery efficiencies, lower cost, and most importantly, safety.
DISCUSSION
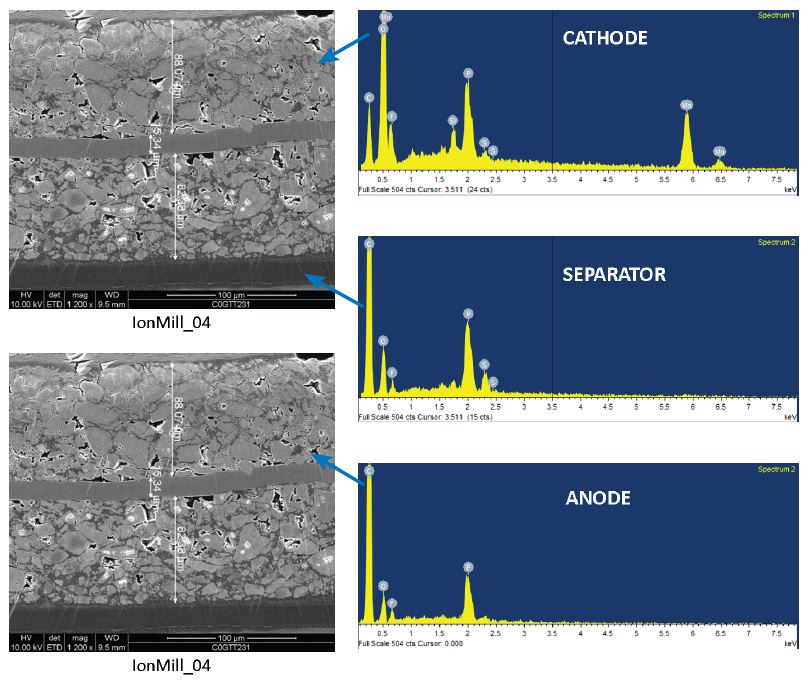
At EAG Laboratories, we provide a suite of techniques suitable for both the structural and chemical characterization of Li-ion batteries. Both scanning and transmission electron microscopy (SEM and TEM) are used to provide the thickness and microstructure of the various layers in the battery. Ion milling techniques are used to preserve the integrity of the sample to assure an accurate representation of the original state of the battery materials. This is essential in order to properly understand process development or battery failures.

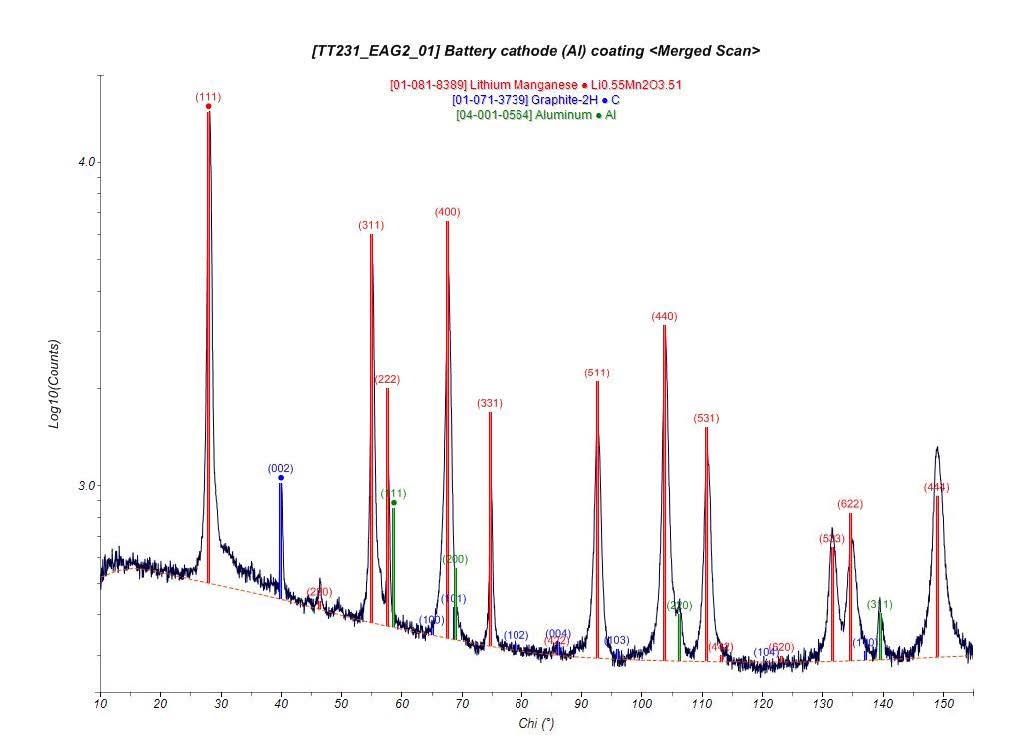
TEM combined with X-Ray Diffraction (XRD) can also be used to analyze the phase transformations associated with the diffusion of Li-ions. Additionally, the thickness of the thin SEI layer caused by electrode-electrolyte interfacial reactions can only be visualized by TEM.
Degradation mechanisms of the battery materials can be analyzed with surface analysis techniques such as X-ray photoelectron spectroscopy (XPS) to detect chemical state information and gas chromatography (GCMS) techniques to detect volatile components that can lead to swelling of the battery. These techniques along with Raman and infrared spectroscopy (FTIR), and Glow Discharge Mass Spectrometry (GDMS) can detect the organic and inorganic species present in the battery, including any impurities that may be present. Inductively coupled optical emission techniques (ICP-OES) can be used to determine the Li/metal ratio to within a 1% uncertainty, which is essential for tuning the cycling stability of Li-ion batteries.
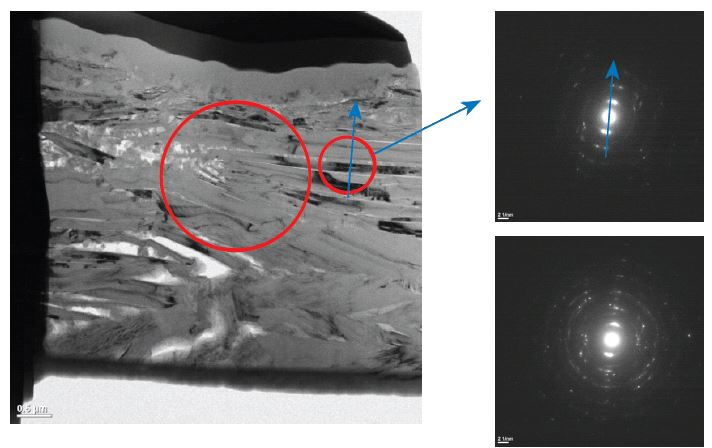
TEM Cross section of a part of the Anode structure shows the stacking of graphite platelets; SAED shows parallel orientation of the platelets.
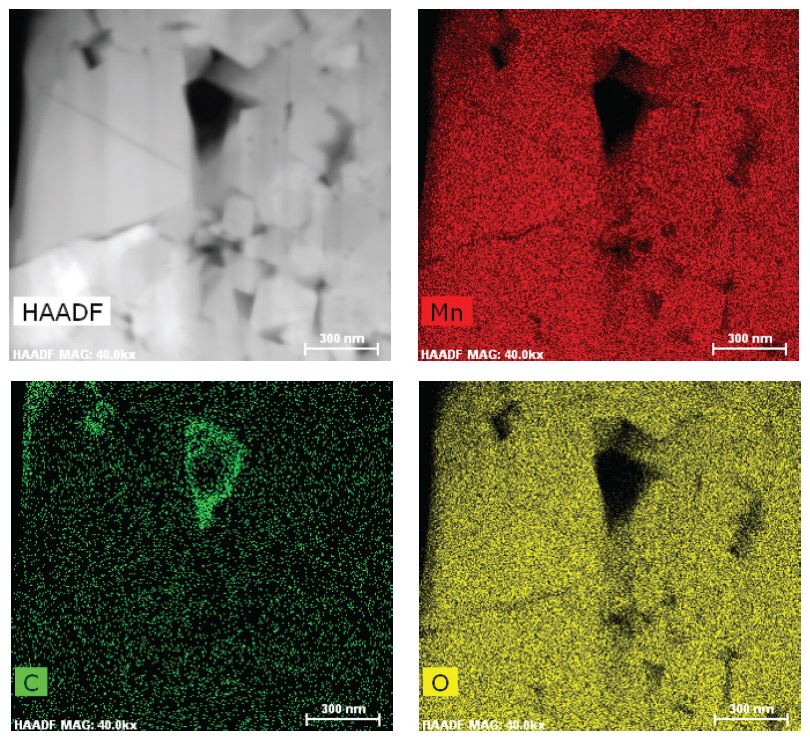
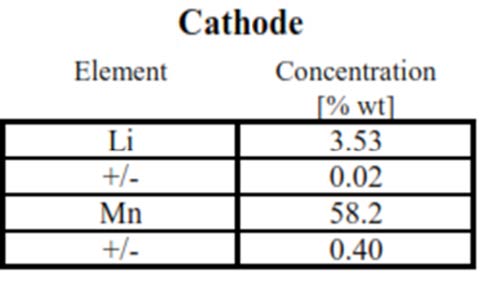
- Battery performance decay is directly related to the loss of electroactive lithium. EAG developed a protocol to extract the electroactive cathode component from battery cells in various charging states or after certain number of charging/discharging cycle.
- ICP-OES technique to accurately determine the elemental composition of the cathode. Being an easily ionized species, lithium assay is challenging and needs to executed with a protocol to factor in this impact .
- Table above shows the extracted cathode composition, including lithium and transition metal content, in this case Mn, as determined by our high performance ICP-OES technique.
- The technique enables one to accurately track the slightest Lithium content change (as lower as 1% relative change).
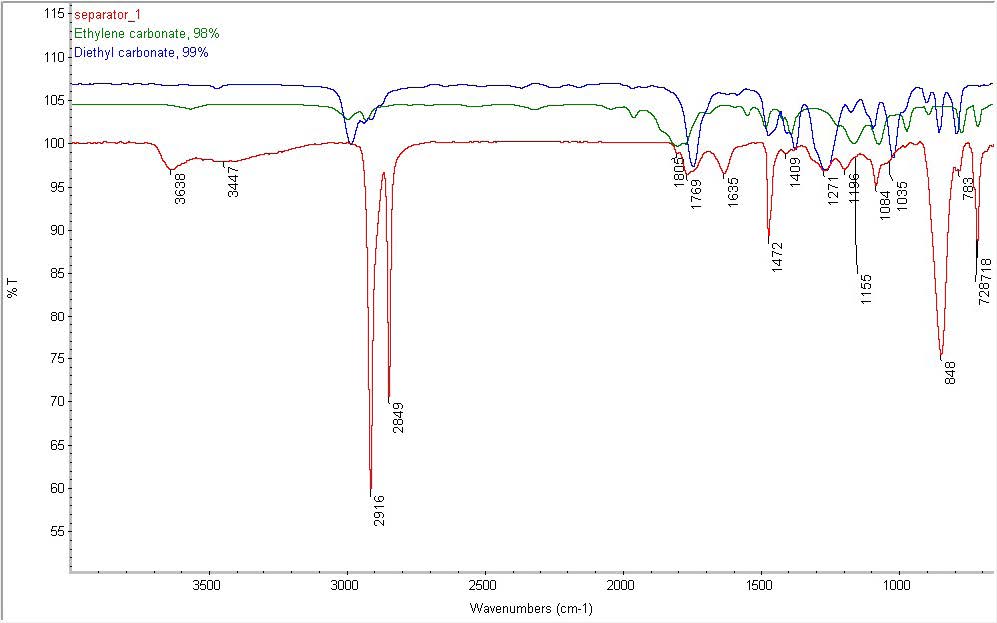
Separator also contains:
- Organic carbonates similar to ethylene and diethyl carbonate
- Amide (1635 cm-1)
- NH and/or OH containing species (3638 and 3447 cm-1)
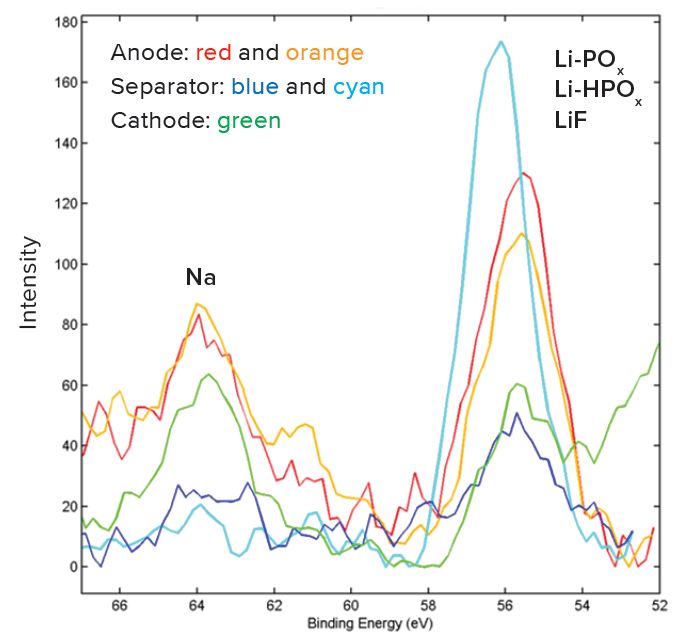
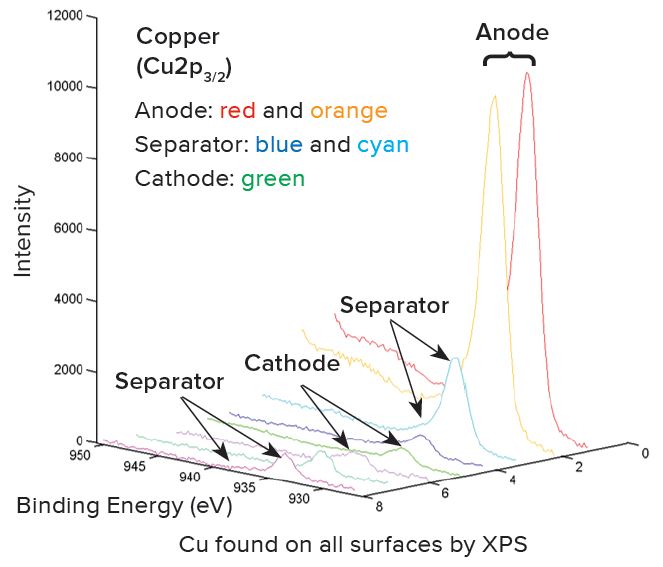
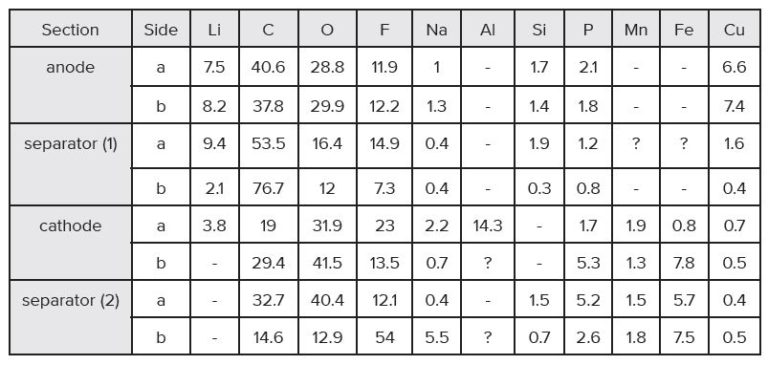
a – Normalized to 100% of the elements detected. XPS does not detect H or He.
b – A dash line “-” indicates the element is not detected.
c – An “x“ indicates that the presence of Li cannot be confirmed or ruled out due to spectral interference from the overlapping Fe3p peak.
d – A question mark “?” indicates species may be present at or near the detection limit of the measurement.
e – Trace amounts of Mg and S were detected in separator (1) and the cathode.
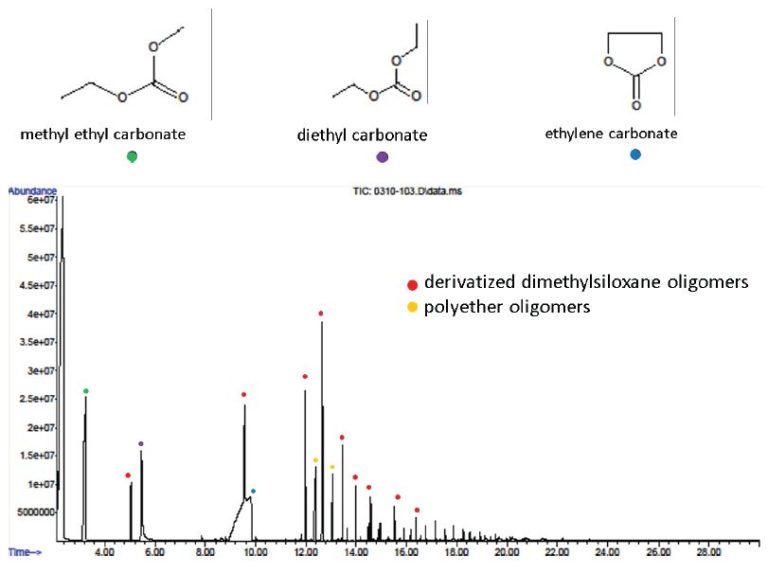
EXPERIMENTAL
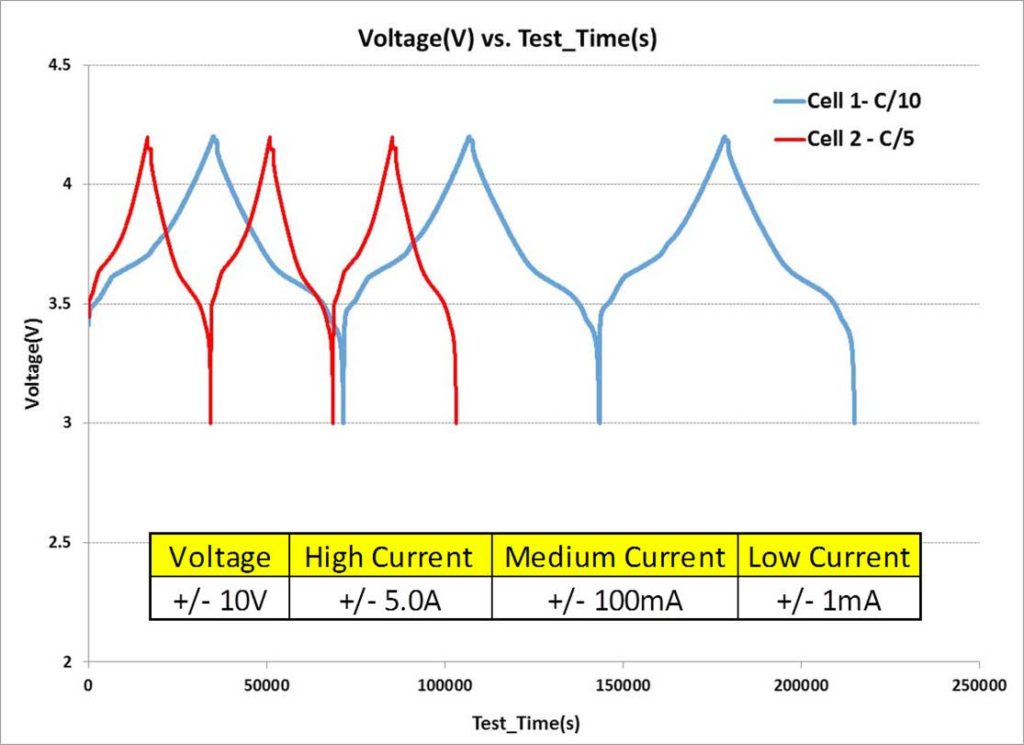
Battery Cycling: Evaluate Charge/discharge of anode and cathode materials at +/- 10V.
FTIR: The two samples analyzed include a separator component and a binder component. The surface of each sample was examined in attenuated total reflection (ATR) mode using a Thermo-Nicolet 6700 Fourier Transform Infrared (FTIR) spectrometer equipped with a Continuum microscope. A Si crystal was used with a typical depth of penetration on the order of 1 micron. The analytical spot size was approximately 100 microns x 100 microns. OMNIC 8.0 software was used to perform data analysis.
XRD: All data was acquired on a Bruker GADDS 2-D area detector with Cr x-ray source (λ=2.28973Å)
SEM: Cross section samples were prepared by ion milling and then coated with Ir to reduce charging.
XPS: X-ray Photoelectron Spectroscopy is used to determine quantitative atomic composition and chemistry. XPS works by irradiating a sample with monochromatic X-rays, resulting in the emission of photoelectrons whose energies are characteristic of the elements and their chemical/oxidation state, and the intensities of which are reflective of the amount of those elements present within the sampling volume. Photoelectrons are generated within the X-ray penetration depth (typically many microns), but only photoelectrons within the top ~50-100Å are detected (see Angle Resolved XPS below for more details). Analyzed region is 1400umx 3000um. Detection limits are approximately 0.05 to 1.0 atomic %.
GCMS: A glass syringe was used to inject tetrahydrofuran “THF” directly into the battery, followed by removal of the solvent with that same syringe. This process was repeated until ~0.5 mL was recovered. The recovered extract was injected directly in the GCMS.
ICP-OES: Inductively Coupled Plasma analytical techniques can quantitatively measure the elemental content of a material from the ppt to the wt% range. Solid samples are dissolved or digested in a liquid, usually an acidic aqueous solution. Solution is then sprayed into the core of and inductively coupled argon plasma, which can reach temperatures of approximately 8000°C. At such high temperature, all analyte species are atomized, ionized and thermally excited, and they can then be detected and quantified with an emission spectrometer (ICP-OES).
Would you like to learn more about Characterization of Li-ion Batteries?
Contact us today for your characterization of Li-ion battery needs. Please complete the form below to have an EAG expert contact you.