Quantification of Bisphenol A
Home » Quantification of Bisphenol A
Bisphenol A (BPA) is a synthetic, organic compound that has the chemical formula (CH3)2C(C6H4OH)2. It is a key monomer used in the synthesis of epoxy resins and polycarbonates plastics. Epoxies, polymers containing cyclic ethers, and polycarbonates, polymers containing esters of carbonic acid, manufactured from BPA possess applications within a multitude of industrial, medical, and food-packaging industries. BPA-based epoxy resins can be found in the lining of water pipes, coatings on the inside of food and beverage packaging, paints and adhesives, sealants, and even dental fillings. Plastics synthesized from BPA can be found in goods such as bottles and sports equipment while also found in medical devices and food packaging.
Both epoxies and polycarbonates can have varying molecular weights depending on the manufacturing conditions. A common occurrence in the manufacturing of BPA-based epoxies or polycarbonates is the monomer not synthesizing completely which would leave some residual BPA monomer behind in the product. A breakdown of the final product can also occur. In the case of a breakdown of the polymer, certain conditions can cause the epoxy or polycarbonate to depolymerize when the material is under harsh environments or with aging during the service life of the material; during breakdown, the polymer “unzips” and becomes a free (i.e., discrete, non-polymerized). Both these occurrences can lead to excess of free BPA existing in the material and then possibly migrating from the epoxy or polycarbonate to other materials the product has been in contact with. It is for these reasons the use of BPA in manufacturing has been the source of debate and discussion in recent years.
As studies aimed at possible health effects of BPA are completed, and their results reviewed by regulatory agencies around the world, the overall safety of this compound is still in up for debate. Taking a look into the recent history of BPA, the European Food Safety Authority (EFSA) had established a tolerable daily intake (TDI) of 5 milligrams per kilogram of body weight per day for BPA in 2002. 1 In January 2007 the EFSA set the TDI to 0.05 milligrams per kilogram of body weight per day due to a reevaluation of uncertainty factors in previously conducted studies.1 However, in October 2008 the United States Food and Drug Administration (FDA) identified BPA’s no-observed-adverse-effect-level of 5 milligrams per kilogram of body weight per day which was the previous level set by the EFSA before reevaluation had occurred.2 Additionally, in July 2012, the FDA banned the use of BPA-based materials used to manufacture baby bottles as well as sippy cups in response to a petition from the American Chemical Council along with a follow up in July 2013 banning BPA-based materials in infant formula packaging .3, 4 The EFSA, in January 2015, reestablishing its new TDI of 4 micrograms per kilogram of body weight per day; however, it was concluded there were no current health concerns from dietary exposure for any persons at the time.5 Both the EFSA and FDA are continuing their investigations as new studies conclude and scientific evidence is collected, evaluated, and compared.6,7
Regardless of industry, testing and evaluation of free BPA in products should not be considered any less vital even though current levels are seen as appropriate. Precise, accurate quantification of BPA in random sampling can provide a manufacturer with valuable information about the raw materials they receive and about their final products they export. International manufacturers of BPA based epoxies and polycarbonates must especially take regular testing into consideration due to the differences in tolerated exposure limits by the different regulatory agencies around the world. As long term studies conclude and the scientific merit evaluated by such regulatory agencies mentioned above, it does not appear the restrictions of BPA will loosen any time soon. Following the trend of the EFSA on their ever tightening TDI and the FDA banning the product in infant-geared products, it would seem that BPA will become more regulated in the future.
BPA Analysis: We Know How
EAG Laboratories has experience in testing a multitude of materials for BPA. It is performed by a proprietary analytical method using High Performance Liquid Chromatograph coupled with a fluorescence detector (HPLC-FLD) for samples being analytically tested that have only a few detectable excipients. If the sample has a complex matrix or contains a multitude of detectable excipients, the use of Liquid Chromatography coupled with mass spectroscopy (LC-MS) or High Resolution Liquid Chromatography coupled with mass spectroscopy (HRLC-MS) can be utilized to analyze the sample. The quantification is accomplished by first utilizing the most appropriate residual monomer extraction technique for the given sample; one that will not hydrolyze the material and makes derivatization unnecessary. Both hydrolyzation and derivatization can cause unreliable data by making the residual monomer concentration increase or decrease from its actual value depending on the exact makeup of the material being tested. The quantitation can also be carried out after a leach and extract technique to test for BPA levels in a material during conditions intended to mimic the products intended use conditions. Secondly, the sample is chromatographically separated to isolate the target analyte above the established limit of detection of the instrument being used.
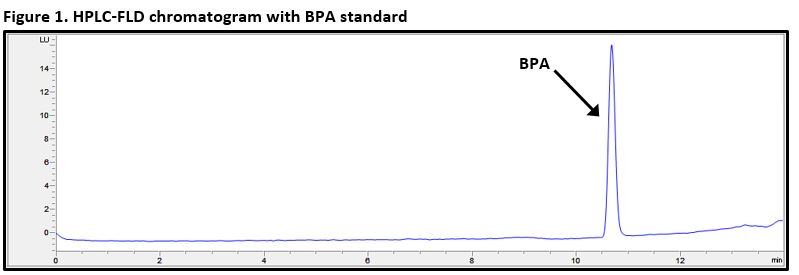
Once the sample has been successfully separated and data collected, the sample can then be compared to a set of prepared standards of known BPA concentrations, prepared in the same fashion. Finally, the sample is compared to the prepared standards in order to determine the concentration found in the testing sample. To ensure all BPA was extracted successfully, a fortified sample is also prepared in which a known amount of BPA is added to the testing sample in order to determine the overall recovery of the analyte from the sample matrix. An example chromatogram of a prepared analytical standard of BPA is presented in Figure 1.
Basics of HPLC-FLD
HPLC is used for separation, identification, and quantitation of a variety of compounds in a variety of matrices. A mixture of solvents or solutions, called the mobile phase, is forced at high pressure through a packed column, usually of coated silica particles, called the stationary phase. Components are separated based on the difference in their affinities for the stationary phase and the mobile phase, and are detected and measured as they elute from the column. The time a chemical component spends in the column from injection until detection is known as retention time, and is an indicator of component identity. As the chemical components leave the column, they are struck by a beam of light which causes the electrons in certain molecules to excite. This excitation causes the molecules to emit light in order to reach a more relaxed state. This emitted light is captured by the detector and processed into a chromatogram as a peak. The measured peak area or height is concentration dependent, and is used to quantitate the component.
Basics of LC-MS
LC/MS combines the techniques of HPLC and MS to characterize the structures of components in a complex matrix. In a LC/MS experiment, the effluent from the HPLC is sent to the ion source of a mass spectrometer for mass analysis of the resolved components. Using an Ion Trap Mass Spectrometer, the parent ions can be characterized by fragmentation, providing structural information for characterization of unknowns, or complete identification of known species by matching both the retention time and fragmentation pattern between the sample and a reference standard.
1 Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to 2,2-BIS(4-HYDROXYPHENYL)PROPANE. EFSA Journal. [Online] 2006, 428, http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.428/epdf (Accessed June 2, 2017).
2 DRAFT ASSESSMENT OF BISPHENOL A FOR USE IN FOOD CONTACT APPLICATIONS. Food and Drug Administration. [Online] 2008, https://www.fda.gov/ohrms/dockets/AC/08/briefing/2008-0038b1_01_02_FDA%20BPA%20Draft%20Assessment.pdf (Accessed June 2, 2017).
3 Indirect Food Additives: Polymers. Department of Health and Human Services. [Online] 2012, 21 CFR Part 177, https://www.federalregister.gov/documents/2012/07/17/2012-17366/indirect-food-additives-polymers (Accessed June 2, 2017).
4 Indirect Food Additives: Adhesives and Components in Coatings. Department of Health and Human Services. [Online] 2013, 21 CFR Part 175, https://www.federalregister.gov/documents/2013/07/12/2013-16684/indirect-food-additives-adhesives-and-components-of-coatings (Accessed June 2, 2017).
5 EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA Journal. [Online] 2017, 13, http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.3978/epdf (Accessed June 2, 2017).
6 Aungst, Jason. Department of Health and Human Services. 2014 Updated safety assessment of Bisphenol A (BPA) for use in food contact applications. [Online] 2014, https://www.fda.gov/downloads/NewsEvents/PublicHealthFocus/UCM424266.pdf (Accessed June 2, 2017)
7 European Food Safety Authority. Bisphenol A: new immune system evidence useful but limited. [Online] 2016, http://www.efsa.europa.eu/en/press/news/161013 (Accessed June 2, 2017)
Would you like to learn more about Quantification of Bisphenol A?
Contact us today for your quantification of Bisphenol A needs. Please complete the form below to have an EAG expert contact you.