Next Steps After Receiving a Cytotoxic Result
Home » Next Steps After Receiving a Cytotoxic Result
Introduction
Biocompatibility assessment protects patients and customers from unforeseen effects from medical devices, consumer products, and materials. Early detection of adverse responses allows companies to rapidly iterate designs to develop safe and high-quality products. However, with some product applications, negative biological responses such as cytotoxicity are inevitable. How a company and CRO approach these results can mean the difference between a product success or failure.
Cytotoxicity Assays
At Eurofins EAG Laboratories, we perform our MEM elution cytotoxicity assay according to ISO 10993-5.1 The assessment begins with the submersion of the product in an extraction vehicle (i.e., solvent) to simulate physiological conditions. This allows toxicants that the patient/consumer could be exposed to elute into the vehicle. The vehicle is subsequently placed on an in vitro culture of healthy mammalian cells. The response of the cells, measured by changes in growth, morphology, metabolic activity, and/or viability, to the extract will reveal the toxic risk of the product. Due to the highly sensitive nature of this test model, unexpected cytotoxic results may occur.
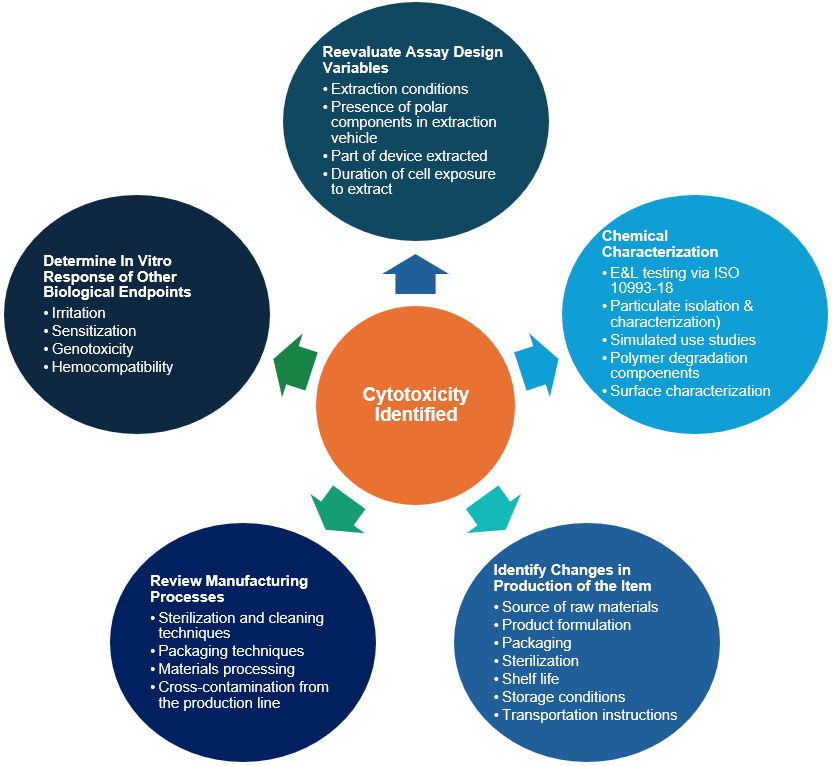
What to do with an Unexpected Result
It can be alarming when a product with known components and extensive planning receives a cytotoxic result. Even if the sample was designed with materials marketed to be biocompatible, minor chemical reactions—from production of the raw materials to the packaging or storage of the product—can introduce cytotoxic contaminants. At this point, the product designer can go multiple directions to address the risk associated with a result.
Would you like to learn more about In Vitro Biocompatibility Testing?
Contact us today! Please complete the form below to have an EAG expert contact you.