Chemical Analysis of Materials Used in Lithium Ion Batteries
Home » Chemical Analysis of Materials Used in Lithium Ion Batteries
In lithium-ion batteries (LIB), energy storage and release are provided by the movement of lithium ions between the cathode and the anode via a suitable medium that is called the electrolyte. In LIB systems, the anode electrode serves as the lithium source and the cathode electrode as the host for lithium ions.
Currently manufactured LIBs are based on variety of chemistries that were developed and selected from wide-ranging variation of suitable materials and electrochemical systems that had been tailored and optimized for specific performance requirements, lifetime needs and, of course, safety (Scheme 1).
It is beyond doubt that LIBs have tremendous potentials for supporting large-scale energy storage technologies. However, manufacturing cost is still a significant barrier to widespread implementation of LIBs especially into diversifications of energy sources for transportation markets. Most of the cost reduction thus far has been achieved by energy density increases. Further cost reductions can be expected through optimization of manufacturing processes. Manufacturing uncertainties arise mainly from fluctuation of processing parameters, but also from the quality of precursors and formulated intermediate products.
Defects introduced by unwanted or unintentional impurities and composition off-stoichiometry, inevitable as the consequence of thermodynamics, are important sources of uncertainties. Like all dynamic systems, the interplay between structure defects and cell performance is strongly coupled. Off-stoichiometry-related crystal defects can destabilize the layered structure of cathode, for instance, thus shortening the cycle life, while impurity defects are known to participate in multiple chemical, physical and electrochemical processes that either accelerate aging or directly lead to failure of batteries:
- Cell shorting due to internal electrical pathways triggered by metallic impurities
- Self-discharging from internal micro-batteries created by magnetic impurities
- Cell explosion triggered by outgassing from impurity catalyzed hydrogen formation and liquid electrolyte decomposition
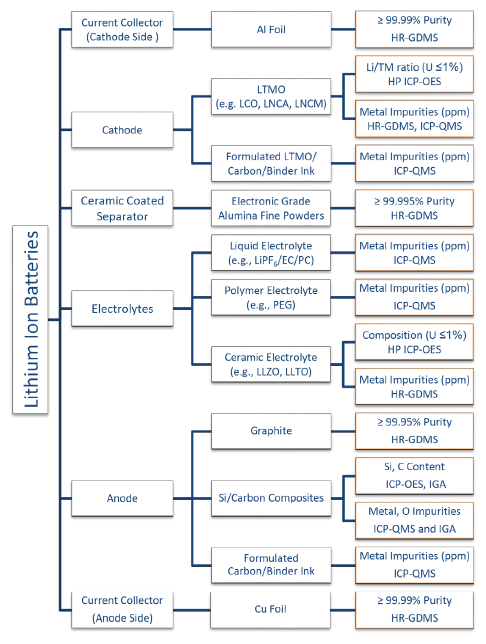
Scheme 1 illustrates some of the chemical analysis techniques and methods that can help to evaluate the full compositions of materials that are currently used for manufacturing LIBs. For each component, we will discuss the sample characteristics, specification requirement, and analytical challenges individually. Accordingly, the choice of the technique and the corresponding analytical characteristics including sampling size, elemental coverage, precision, accuracy, and limits of detection is going to be discussed.
Would you like to learn more about Chemical Analysis of Lithium Ion Batteries?
Contact us today for your chemical analysis of materials used in Lithium Ion Batteries. Please complete the form below to have an EAG expert contact you.